Acetate-derived Alkaloids
The poison hemlock (Conium maculatum; Umbelliferae/ Apiaceae) accumulates a range of simple piperidine alkaloids, e.g. coniine and γ-coniceine (Figure 115). These alkaloids would appear to be related to simple lysine-derived compounds such as pelletierine, but, surprisingly, a study of their biosynthetic origins excluded lysine as a precursor, and demonstrated the sequence shown in Figure 115 to be operative. A fatty acid precursor, capric (octanoic) acid, is utilized, and this is transformed into the ketoaldehyd by successive oxidation and reduction steps. This ketoaldehyde is then the substrate for a transamination reaction, the amino group originating from L-alanine. Subsequent transformations are imine formation giving the heterocyclic ring of γ-coniceine, and then reduction to coniine. Pinidine(Figure 116) from Pinus species is found to have a rather similar origin in acetate, and most likely a poly-β-keto acid. During the sequence outlined in Figure 116, the carboxyl group is lost. Note that an alternative folding of the poly-β-keto acid and loss of carboxyl might be formulated.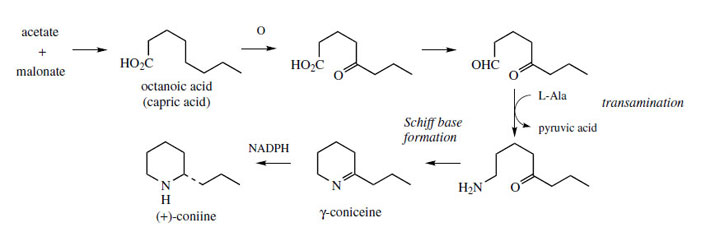 |
| Figure 115 |
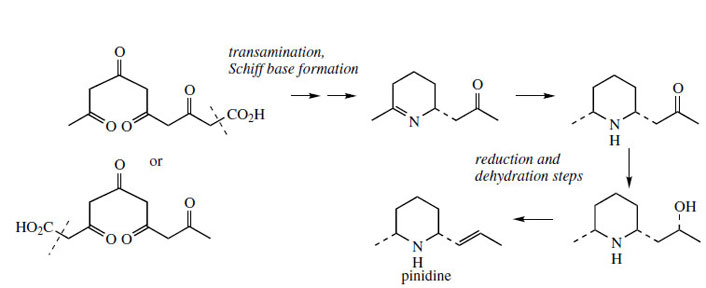 |
| Figure 116 |
Conium maculatum
Conium maculatum (Umbelliferae/Apiaceae) or poison hemlock is a large biennial herb indigenous to Europe and naturalized in North and South America. As a common poisonous plant, recognition is important, and this plant can be differentiated from most other members of the Umbelliferae/Apiaceae by its smooth purple-spotted stem. The dried unripe fruits were formerly used as a pain reliever and sedative, but have no medicinal use now. The ancient Greeks are said to have executed condemned prisoners, including Socrates, using poison hemlock. The poison causes gradual muscular paralysis followed by convulsions and death from respiratory paralysis. All parts of the plant are poisonous due to the alkaloid content, though the highest concentration of alkaloids is found in the green fruit (up to 1.6%). The major alkaloid (about 90%) is the volatile liquid coniine (Figure 115), with smaller amounts of structurally related piperidine alkaloids, including N-methylconiine and ?-coniceine (Figure 115).
In North America, the name hemlock refers to species of Tsuga (Pinaceae), a group of coniferous trees, which should not be confused with the poison hemlock.




