Piperidine Alkaloids
N-Methylpelletierine (Figure 22) is an alkaloidal constituent of the bark of pomegranate (Punica granatum; Punicacae), where it occurs with pelletierine and pseudopelletierine (Figure 22), the mixture of alkaloids having activity against intestinal tapeworms. NMethylpelletierine and pseudopelletierine are homologues of hygrine and tropinone respectively, and a pathway similar to Figure 3 using the diamine cadaverine (Figure 22) may be proposed. (The rather distinctive names cadaverine and putrescine reflect early isolations of these compounds from decomposing animal flesh.) In practice, the Mannich reaction involving the Δ1-piperidinium salt utilizes the more nucleophilic acetoacetyl-CoA rather than acetyl- CoA, and the carboxyl carbon from acetoacetate appears to be lost during the reaction by suitable hydrolysis/decarboxylation reactions (Figure 22). Anaferine (Figure 22) is an analogue of cuscohygrine in which a further piperidine ring is added via an intermolecular Mannich reaction.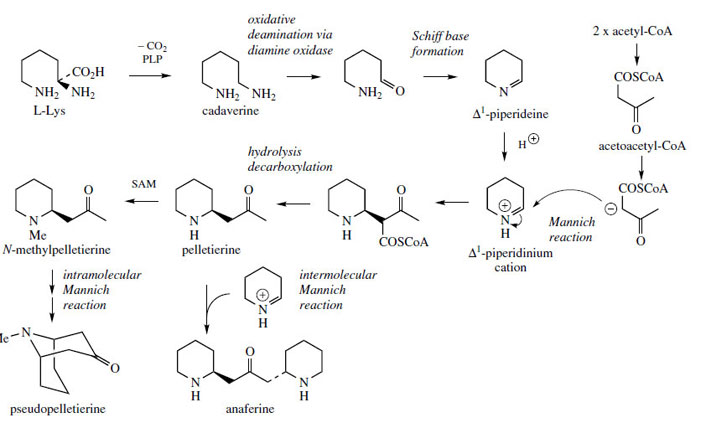 |
| Figure 22 |
he alkaloids found in the antiasthmatic plant Lobelia inflata* (Campanulaceae) contain piperidine rings with alternative C6C2 side-chains derived from phenylalanine via cinnamic acid. These alkaloids are produced as in Figure 23, in which benzoylacetyl-CoA, an intermediate in the β-oxidation of cinnamic acid provides the nucleophile for the Mannich reaction. Oxidation in the piperidine ring gives a new iminium species, and this can react further with a second molecule of benzoylacetyl-CoA, againvia a Mannich reaction. Naturally, because of the nature of the side-chain, the second intramolecular Mannich reaction, as involved in pseudopelletierine biosynthesis, is not feasible. Alkaloids such as lobeline and lobelanine from Lobelia inflata, or sedamine from Sedum acre (Crassulaceae), are products from further N-methylation and/or carbonyl reduction reactions (Figure 23).
The simple piperidine alkaloid coniine from poison hemlock is not derived from lysine, but originates by an amination process and is discussed on page 381.
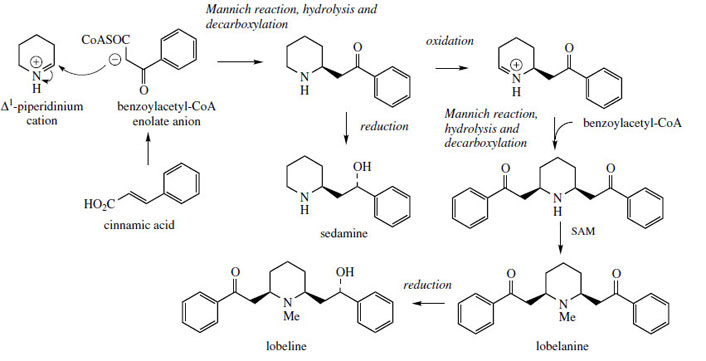 |
| Figure 23 |
The pungency of the fruits of black pepper (Piper nigrum; Piperaceae), a widely used condiment, is mainly due to the piperidine alkaloid piperine (Figure 24). In this structure, the piperidine ring forms part of a tertiary amide structure, and is incorporated via piperidine itself, the reduction product of Δ1-piperideine (Figure 22). The piperic acid portion is derived from a cinnamoyl-CoA precursor, with chain extension using acetate/malonate (compare flavonoids, page 149), and combines as its CoA ester with piperidine.
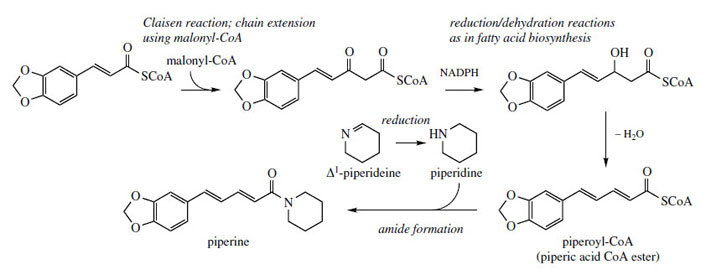 |
| Figure 24 |
Lobelia Lobelia or Indian tobacco consists of the dried leaves and tops of Lobelia inflata(Campanulaceae), an annual herb from the USA and Canada. Lobelia contains about 0.2-0.4% of alkaloids, of which the piperidine derivative lobeline (Figure 23) is the chief constituent. Minor alkaloids identified include closely related structures, e.g. lobelanine (Figure 23). The North American Indians employed lobelia as an alternative or substitute for tobacco (Nicotiana tabacum; Solanaceae), and it is found that lobeline stimulates nicotinic receptor sites in a similar way to nicotine, but with a weaker effect. Lobeline has been employed in preparations intended as smoking deterrents. The crude plant drug has also long been used to relieve asthma and bronchitis, though in large doses it can be quite toxic. |




