Amaryllidaceae Alkaloids
Various types of alkaloid structure are encountered in the daffodil family, the Amaryllidaceae, and they can be rationalized better through biosynthesis than by structural type. The alkaloids arise by alternative modes of oxidative coupling of precursors related to norbelladine (Figure 69), which is formed through combination of 3,4- dihydroxybenzaldehyde with tyramine, these two preursors arising from phenylalanine and tyrosine respectively. Three structural types of alkaloid can be related to 4*-O-methylnorbelladine by different alignments of the phenol rings, allowing coupling para–ortho (A), para–para (B), or ortho–para (C) as shown in Figure 69. For galanthamine, the dienone formed via oxidative coupling (C) undergoes nucleophilic addition from the phenol group, forming an ether linkage (compare opium alkaloids, page 328), and the sequence is completed by reduction and methylation reactions. For lycorine and crinine, although details are not given in Figure 69, it is apparent that the nitrogen atom acts as a nucleophile towards the dienone system in a similar manner, generating the new heterocyclic ring systems. Alkaloids such as lycorine, crinine, and galanthamine can undergo further modifications, which include ring cleavage reactions, generating many more variations than can be considered here.The Amaryllidaceae family includes Amaryllis, Narcissus, and Galanthus, and the alkaloid content of bulbs from most members makes these toxic. Lycorine was first isolated from Lycorus radiata, but is common and found throughout the family.
Galanthaminefrom snowdrops (Galanthus species) is currently an important drug material of value in treating Alzheimer’s disease.
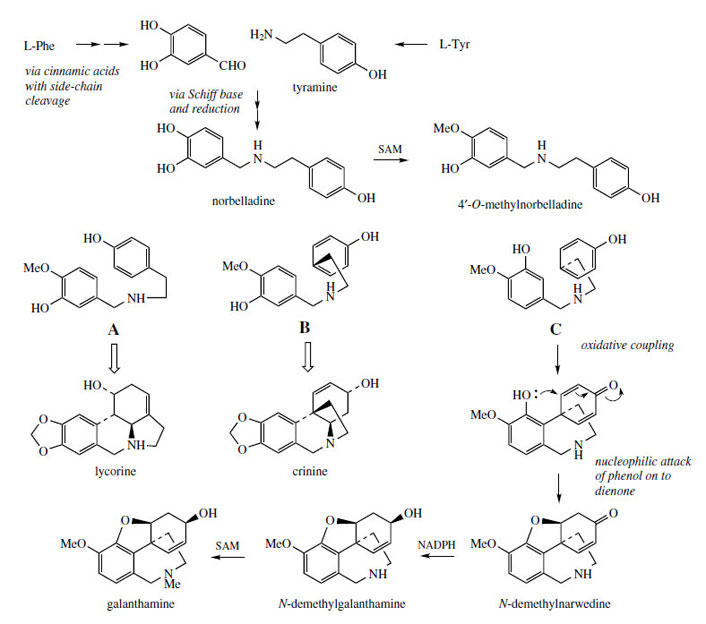 |
| Figure 69 |
Galanthamine
Galantamine (galanthamine) can be isolated from a number of species of the Amaryllidaceae, including snowdrops (Galanthus species), daffodils (Narcissus pseudonarcissus), and snowflakes (Leucojum species), where typical content varies from about 0.05 to 0.2% in the bulbs. It is currently isolated for drug use from the bulbs of wild Leucojum aestivum and Galanthus species, since commercial synthesis is not economic. Galantamine acts as a competitive cholinesterase inhibitor, and enhances cognitive function in the treatment of Alzheimer's disease by raising acetylcholine levels in brain areas lacking cholinergic neurones. In common with other treatments for Alzheimer's disease, it does not cure the condition, but merely slows the rate of cognitive decline




