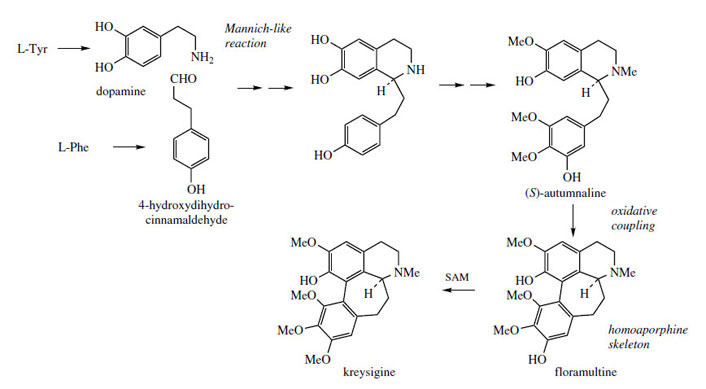
Phenethylisoquinoline Alkaloids
Several genera in the lily family (Liliaceae) are
found to synthesize analogues of the benzyltetrahydroisoquinoline
alkaloids, e.g. autumnaline (Figure 65), which contain an extra carbon
between the tetrahydroisoquinoline and the pendant
aromatic rings. This skeleton is formed in
a similar way to that in the benzyltetrahydroisoquinolines
(Figure 65), but a whole C6C3 unit rather
than a C6C2 fragment functions as the reacting
aldehyde. Typically, dopamine (from tyrosine)
and 4-hydroxydihydrocinnamaldehyde (from
phenylalanine) are involved in the initial condensation,
and further hydroxylation and methylation
steps then build up the substitution pattern to
that of autumnaline. Phenolic oxidative coupling
accounts for the occurrence of homoaporphine
alkaloids such as floramultine and kreysigine in Kreysigia multiflora (Liliaceae/Convallariaceae).
(S )-Autumnaline has also been found to act
as a precursor for colchicine (Figure 66), an
alkaloid containing an unusual tropolone ring. |
 |
| Figure 65 |
 |
| Figure 66 |
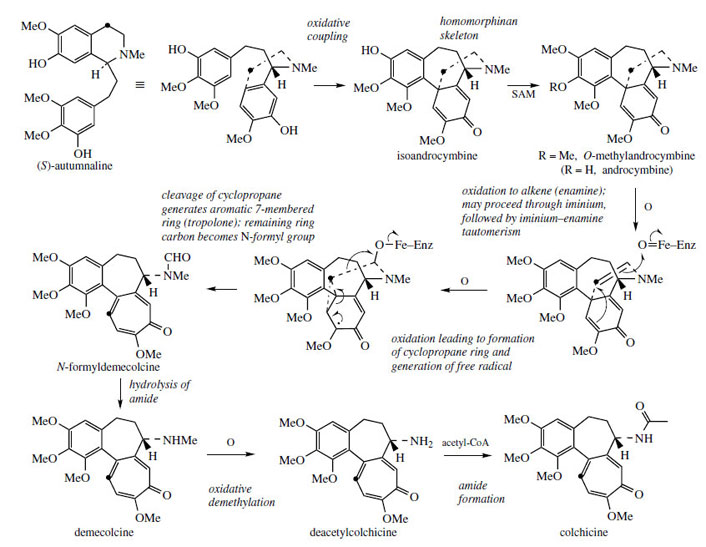
Colchicine is found in species of Colchicum*, e.g.Colchicum autumnale (Liliaceae/Colchicaceae), as well as many other plants in the Liliaceae.Colchicine no longer has its nitrogen atom in a ring system, and extensive reorganization of the autumnaline structure is thus necessary. The seven-membered tropolone ring was shown by labelling experiments to originate by ring expansion of the tyrosine-derived aromatic ring taking in the adjacent benzylic carbon (Figure 66). Prior to these remarkable rearrangements, oxidative coupling of autumnaline in the para–parasense features in the pathway giving the dienone isoandrocymbine, which has a homomorphinan skeleton (compare salutaridine, Figure 50). The isomer androcymbine (Figure 66) had been isolated from Androcymbium melanthioides (Liliaceae/ Colchicaceae), thus giving a clue to the biosynthetic pathway. Methylation follows giving O-methylandrocymbine, and it is then proposed that enzymic oxidation to an enamine yields the substrate for ring modification. Experimental labelling studies are then best explained by formation of a cyclopropane ring followed by ring opening to generate the 6π electron aromatic tropolone system, incorporating the original tyrosine benzylic carbon into the seven-membered ring, and also breaking the original phenylethylamine sidechain between the carbons. One carbon is left on the nitrogen as a formyl group, and this can be lost by hydrolysis. Colchicine is produced by exchanging the N-methyl group for an N-acetyl group, by way of an oxidative demethylation followed by acetylation using acetyl-CoA. Demecolcine and deacetylcolchicine are intermediates in the process.
Colchicum
Colchicum seed and corm are obtained from Colchicum autumnale (Liliaceae/Colchicaceae), the autumn crocus or meadow saffron. The plant, though not a crocus, produces crocus-like flowers in the autumn, the leaves not emerging until the spring. It is a native of Europe, is widely cultivated as an ornamental garden plant, and is grown for drug use, mainly in Europe and North Africa. The principal alkaloid is colchicine (Figure 66), which occurs to the level of about 0.8% in the seed, and 0.6% in the corm. As an N-acetyl derivative, colchicine does not display any significant basicity, and does not form well-defined salts. Demecolcine (N-deacetyl-N-methylcolchicine) (Figure 66) is a minor constituent in both corm and seeds.
Extracts of Colchicum autumnale, and later colchicine itself, have been used in the treatment of gout, a painful condition in which impaired purine metabolism leads to a build-up of uric acid crystals in the joints. Colchicine is an effective treatment for acute attacks, but it is very toxic, and this restricts its general use. It appears to act primarily as an anti-inflammatory agent, and does not itself affect uric acid metabolism, which needs to be treated with other agents, e.g. a xanthine oxidase inhibitor such as allopurinol. The cytotoxic properties of colchicine and related alkaloid structures from C. autumnale led to their being tested as potential anticancer agents, though they still proved too toxic for medicinal use. Colchicine binds to tubulin in the mitotic spindle, preventing polymerization and assembly into microtubules as do podophyllotoxin and vincristine, and is a useful biochemical probe. However, the ability of colchicine to act as a mitotic poison is exploited in plant breeding, since the interference with mitosis results in multiplication of chromosomes in the cell nucleus without the process of cell division. Cell division recommences on cessation of treatment. This allows the generation of mutations (polyploids) and possible new varieties of plant. Colchicine is also found in other species of Colchicum, as well as many other plants in the Liliaceae (e.g. Bulbocodium, Gloriosa, Merendera, and Sandersonia), a group of plants now classified as the family Colchicaceae.




