Pyrroloindole Alkaloids
Both C-2 and C-3 of the indole ring can be regarded as nucleophilic, but reactions involving C-2 appear to be the most common in alkaloid biosynthesis. There are examples where the nucleophilic character of C-3 is exploited, however, and the rare pyrroloindole skeleton typified by physostigmine (eserine) (Figure 95) is a likely case. A suggested pathway to physostigmine is by C-3 methylation of tryptamine, followed by ring formation involving attack of the primary amine function on to the iminium ion (Figure 95). Further substitution is then necessary. Dimers with this ring system are also known, e.g. chimonanthine (Figure 95) from Chimonanthus fragrans (Calycanthaceae), the point of coupling being C-3 of the indole, and an analogous radical reaction may be proposed. Physostigmine is found in seeds of Physostigma venenosum* (Leguminosae/Fabaceae) and has played an important role in pharmacology because of its anticholinesterase activity. The inherent activity is in fact derived from the carbamate side-chain rather than the heterocyclic ring system, and this has led to a range of synthetic materials being developed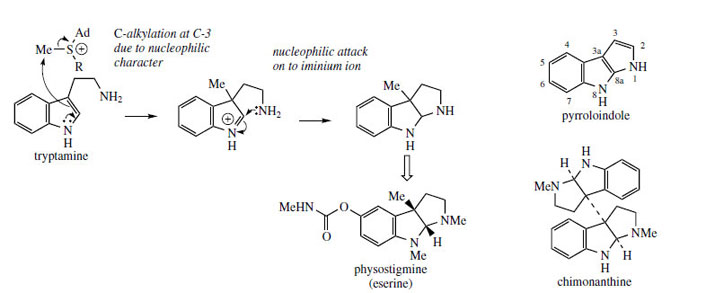 |
| Figure 95 |
Camptothecin
Camptothecin (Figure 93) and derivatives are obtained from the Chinese tree Camptotheca acuminata (Nyssaceae). Seeds yield about 0.3% camptothecin, bark about 0.2%, and leaves up to 0.4%. Camptotheca acuminata is found only in Tibet and West China, but other sources of camptothecin such as Nothapodytes foetida (formerly Mappia foetida) (Icacinaceae), Merilliodendron megacarpum (Icacinaceae), Pyrenacantha klaineana (Icacinaceae), Ophiorrhiza mungos (Rubiaceae), and Ervatmia heyneana (Apocynaceae) have been discovered. In limited clinical trials camptothecin showed broad-spectrum anticancer activity, but toxicity and poor solubility were problems. The natural 10- hydroxycamptothecin (about 0.05% in the bark of C. acuminata) is more active than camptothecin, and is used in China against cancers of the neck and head. Synthetic analogues 9-aminocamptothecin (Figure 94) and the water-soluble derivatives topotecanand irinotecan (Figure 94) showed good responses in a number of cancers; topotecan and irinotecan are now available for the treatment of ovarian cancer and colorectal cancer, respectively. Irinotecan is a carbamate pro-drug of 10-hydroxy-7-ethylcamptothecin, and is converted into the active drug by liver enzymes. These agents act by inhibition of the enzyme topoisomerase I, which is involved in DNA replication and reassembly, by binding to and stabilizing a covalent DNA-topoisomerase complex. Camptothecin has also been shown to have potentially useful activity against pathogenic protozoa such as Trypanosoma brucei and Leishmania donovani, which cause sleeping sickness and leishmaniasis respectively. Again, this is due to topoisomerase I inhibition.
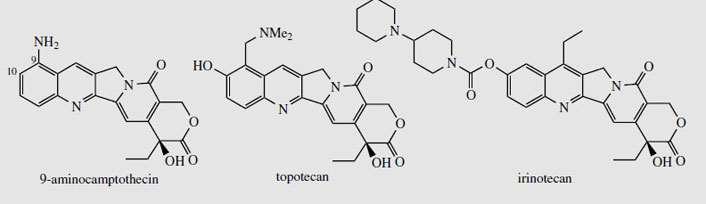 |
| Figure 94 |
Physostigma
Physostigma venenosum (Leguminosae/Fabaceae) is a perennial woody climbing plant found on the banks of streams inWest Africa. The seeds are known as Calabar beans (from Calabar, now part of Nigeria) and have an interesting history in the native culture as an ordeal poison. The accused was forced to swallow a potion of the ground seeds, and if the mixture was subsequently vomited, he/she was judged innocent and set free. If the poison took effect, the prisoner suffered progressive paralysis and died from cardiac and respiratory failure. It is said that slow consumption allows the poison to take effect, whilst emesis is induced by a rapid ingestion of the dose.
 |
| Figure 96 |
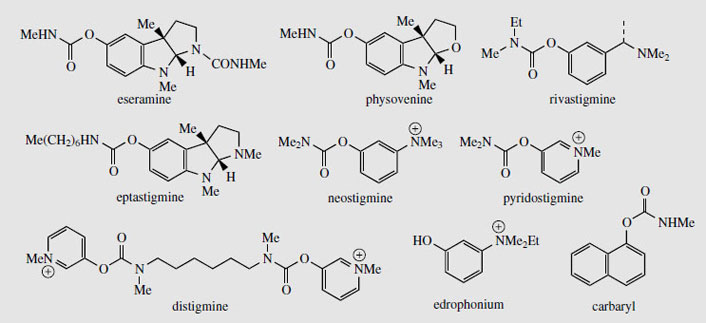
The seeds contain several alkaloids (alkaloid content about 1.5%), the major one (up to 0.3%) being physostigmine (eserine) (Figure 95). The unusual pyrroloindole ring system is also present in some of the minor alkaloids, e.g. eseramine (Figure 96), whilst physovenine (Figure 96) contains an undoubtedly related furanoindole system. Another alkaloid, geneserine (Figure 97), is an artefact produced by oxidation of physostigmine, incorporating oxygen into the ring system, probably by formation of an N-oxide and ring expansion. Solutions of physostigmine are not particularly stable in the presence of air and light, especially under alkaline conditions, oxidizing to a red quinone, rubeserine (Figure 97).
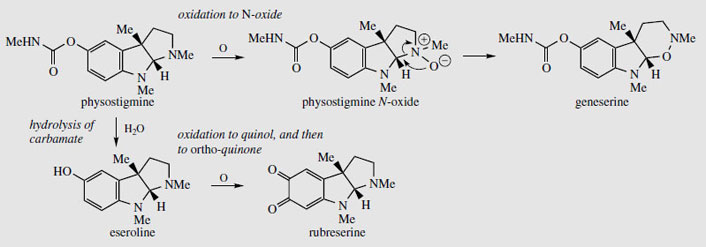 |
| Figure 97 |
Physostigmine (eserine) is a reversible inhibitor of cholinesterase, preventing normal destruction of acetylcholine and thus enhancing cholinergic activity. Itsmajor use is as a miotic, to contract the pupil of the eye, often to combat the effect of mydriatics such as atropine. It also reduces intraocular pressure in the eye by increasing outflow of the aqueous humour, and is a valuable treatment for glaucoma, often in combination with pilocarpine. Because it prolongs the effect of endogenous acetylcholine, physostigmine can be used as an antidote to anticholinergic poisons such as hyoscyamine/atropine, and it also reverses the effects of competitive muscle relaxants such as curare, tubocurarine, atracurium, etc. Anticholinesterase drugs are also of value in the treatment of Alzheimer's disease, which is characterized by a dramatic decrease in functionality of the central cholinergic system. Use of acetylcholinesterase inhibitors can result in significant memory enhancement in patients, and analogues of physostigmine are presently in use (e.g.rivastigmine) or in advanced clinical trials (e.g. eptastigmine (Figure 96)). These analogues have a longer duration of action and less toxicity than physostigmine.
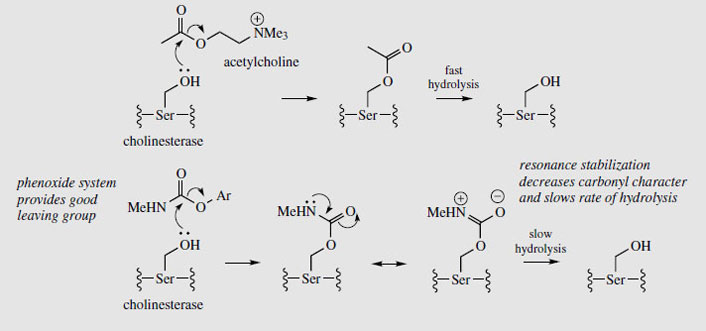 |
| Figure 98 |
The biological activity of physostigmine resides primarily in the carbamate portion, which is transferred to the hydroxyl group of an active site serine in cholinesterase (Figure 98). The enzyme is only slowly regenerated by hydrolysis of this group, since resonance contributions reduce the reactivity of the carbonyl in the amide relative to the ester. Accordingly, cholinesterase becomes temporarily inactivated. Synthetic analogues of physostigmine which have been developed retain the carbamate residue, an aromatic ring to achieve binding and to provide a good leaving group, whilst ensuring water-solubility through possession of a quaternary ammonium system. Neostigmine, pyridostigmine, and distigmine (Figure 96) are examples of synthetic anticholinesterase drugs used primarily for enhancing neuromuscular transmission in the rare autoimmune condition myasthenia gravis, in which muscle weakness is caused by faulty transmission of nerve impulses. Edrophonium is a short-acting competitive blocker of the acetylcholinesterase active site, which is used to help diagnose myasthenia gravis. A number of carbamate insecticides, e.g. carbaryl (Figure 96), also depend on inhibition of cholinesterase for their action, insect acetylcholinesterase being more susceptible to such agents than the mammalian enzyme. Physostigmine displays little insecticidal action because of its poor lipid solubility.




