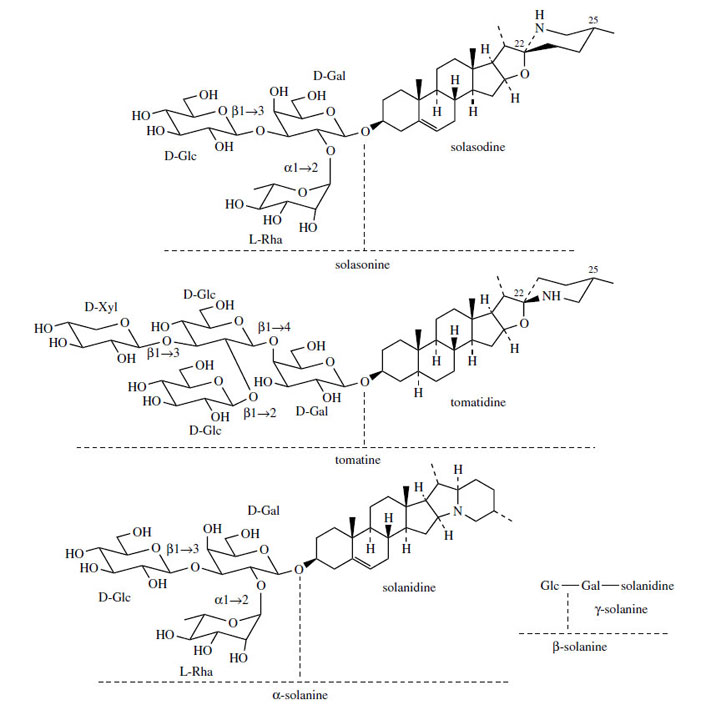
Steroidal Alkaloids
Many plants in the Solanaceae accumulate steroidal
alkaloids based on a C27 cholestane skeleton, e.g.
solasodine and tomatidine (Figure 126). These
are essentially nitrogen analogues of steroidal
saponins and have already been
briefly considered along with these compounds.
In contrast to the oxygen analogues, these compounds
all have the same stereochemistry at
C-25 (methyl always equatorial), but C-22 isomers
do exist, as solasodine and tomatidine exemplify.
They are usually present as glycosides which
have surface activity and haemolytic properties
as do the saponins, but these compounds are
also toxic if ingested. Solasonine from Solanumspecies and tomatine (Figure 126) from tomato (Lycopersicon esculente) are typical examples of
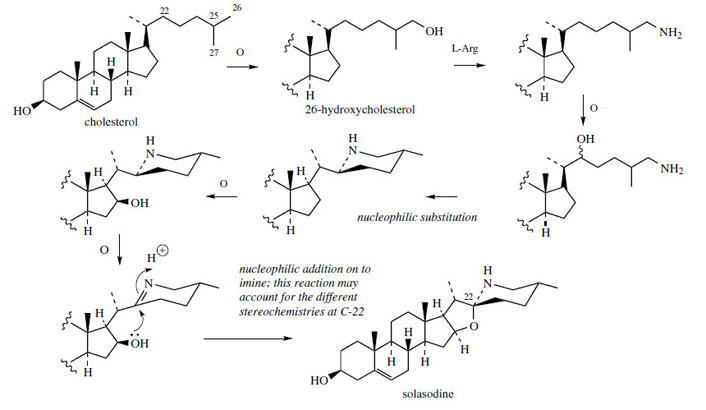
such glycosides.As with the sapogenins, this group of steroidal
alkaloids is derived from cholesterol, with appropriate
side-chain modifications during the sequence
(Figure 127). |
Amination appears to employ
L-arginine as the nitrogen source, probably via a
substitution process on 26-hydroxycholesterol. A
second substitution allows 26-amino-22-hydroxycholesterol
to cyclize, generating a piperidine ring.
After 16β-hydroxylation, the secondary amine is
oxidized to an imine, and the spiro-system can
be envisaged as the result of a nucleophilic
addition of the 16β-hydroxyl on to the imine
(or iminium via protonation). Whether the 22R
(as in solasodine) or 22S (as in tomatidine)
configuration is established may depend on this
reaction.
 |
| Figure 126 |
A variant on the way the cholesterol sidechain
is cyclized can be found in solanidine(Figure 126), which contains a condensed ring
system with nitrogen at the bridgehead. Solanidine
is found in potatoes (Solanum tuberosum), typically
as the glycoside α-solanine (Figure 126).
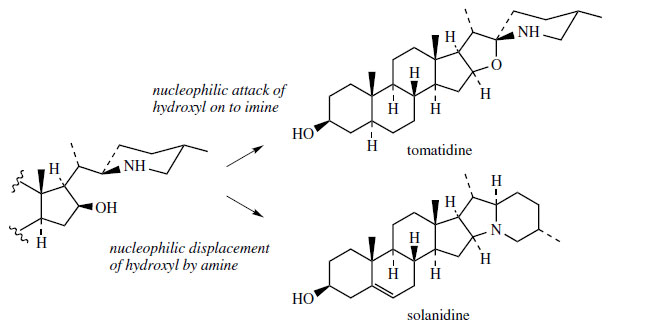
This condensed ring system appears to be produces
by a branch from the main pathway to solasodine/tomatidine structures. Thus, a substitution process
will allow generation of the new ring system
(Figure 128).
Since the production of medicinal steroids from
steroidal saponins requires preliminary
degradation to remove the ring systems containing
the original cholesterol side-chain, it is
immaterial whether these rings contain oxygen or
nitrogen. Thus, plants rich in solasodine or tomatidinecould also be employed for commercial steroid production. Similarly, other Solanum alkaloids*
such as solanidine with nitrogen in a condensed
ring system might also be exploited.
 |
| Figure 127 |
 |
| Figure 128 |
Several plants in the Liliaceae, notably the
genus Veratrum (Liliaceae/Melanthiaceae), contain
a remarkable group of steroidal alkaloids in
which a fundamental change to the basic steroid
nucleus has taken place. This change expands
ring D by one carbon at the expense of ring
C, which consequently becomes five-membered. The resulting skeleton is termed a C-nor-Dhomosteroid
in keeping with these alterations in
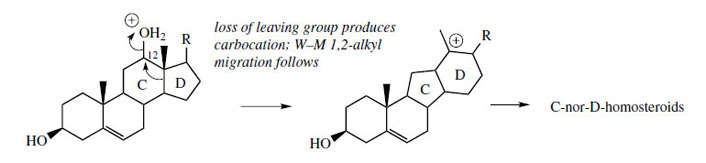
ring size. Cholesterol is a precursor of this group of
alkaloids, and a mechanism accounting for the ring
modifications is shown in Figure 129, where the
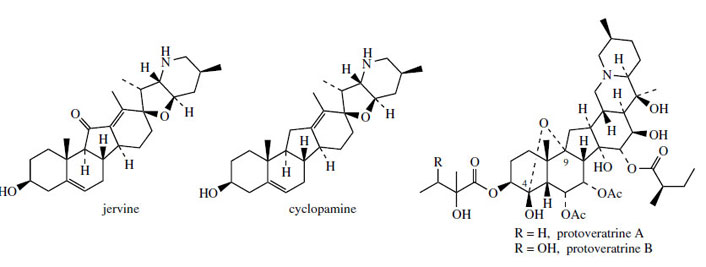
changes are initiated by loss of a suitable leaving group from C-12. Typical representatives of Cnor- D-homosteroids are jervine and cyclopamine (Figure 130) from Veratrum californicum, toxic components in this plant that are responsible
for severe teratogenic effects. Animals grazing
on V. californicum and some other species of
Veratrum frequently give birth to young with
cyclopia, a malformation characterized by a single
eye in the centre of the forehead. The teratogenic
effects of jervine, cyclopamine, and cyclopamine glucoside (cycloposine) on the developing fetus
have now been well established. Other Veratrumalkaloids, especially those found in V. album
and V. viride, have been employed medicinally
as hypotensive agents, and used in the same
way as Rauwolfia alkaloids,
often in combination with Rauwolfia. These
medicinal alkaloids, e.g. protoveratrine A and
protoveratrine B (Figure 130), which are esters
of protoverine, are characterized by fusion of two more six-membered rings on to the C-nor-
D-homosteroid skeleton. This hexacyclic system
is extensively oxygenated, and a novel hemiketal
linkage bridges C-9 with C-4. Both the jervine
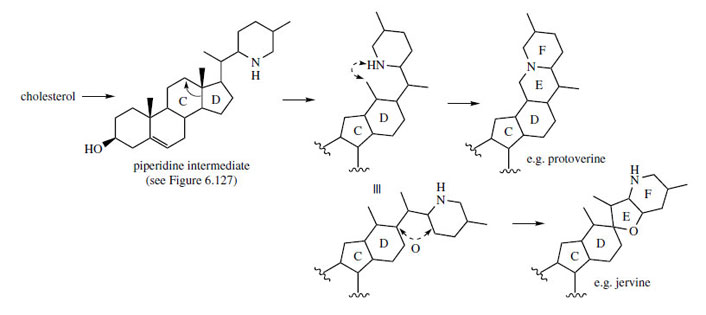
and protoverine skeletons are readily rationalized
through additional cyclization reactions involving
a piperidine ring, probably formed by processes
analogous to those seen with the Solanum alkaloids
(Figure 127). These are outlined in Figure 131,
which suggests the participation of the piperidine intermediate from Figure 127. Typically, both
types of alkaloid are found co-occurring in
Veratrum species.
 |
| Figure 129 |
 |
| Figure 130 |
 |
| Figure 131 |
 |
| Figure 132 |
Many steroidal derivatives are formed by
truncation of the original C8 side-chain, and C21 pregnane derivatives are important animal hormones
or intermediates on the
way to other natural steroidal derivatives, e.g.
cardioactive glycosides. Alkaloids
based on a pregnane skeleton are found in plants, particularly in the Apocynaceae and Buxaceae, and
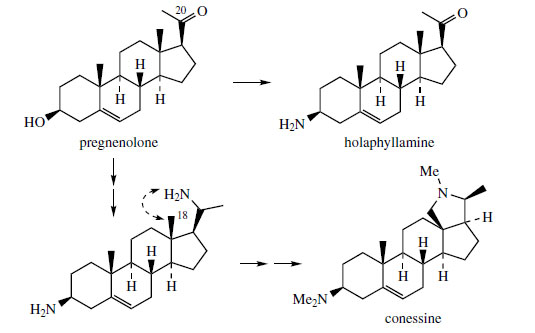
pregnenolone (Figure 132) is usually involved
in their production. Holaphyllamine from Holarrhena
floribunda (Apocynaceae) is obtained from
pregnenolone by replacement of the 3-hydroxyl
with an amino group (Figure 132). Conessine(Figure 132) from Holarrhena antidysenterica is
also derived from pregnenolone, and requires two
amination reactions, one at C-3 as for holaphyllamine,
plus a further one, originally at C-20, probably
via the C-20 alcohol. The new ring system in
conessine is then the result of attack of the C-20
amine on to the C-18 methyl, suitably activated,
of course. The bark of H. antidysenterica has long
been used, especially in India, as a treatment for
amoebic dysentery.
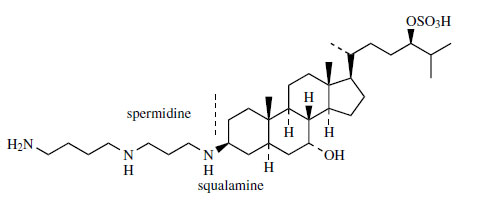
The novel steroidal polyamine squalamine (Figure 133) has been isolated in very small amounts (about 0.001%) from the liver of the dogfish shark (Squalus acanthias), and is attracting attention because of its remarkable
antimicrobial activity. This compound is a broadspectrum
agent effective at very low concentrations
against Gram-positive and Gram-negative bacteria,
and also fungi, protozoa, and viruses including
HIV. The sulphated side-chain helps to make
squalamine water soluble. The polyamine portion
is spermidine, a compound widely distributed
in both animals and plants. Related aminosterol
derivatives with similar high antimicrobial activity
have also been isolated from the liver extracts.
 |
| Figure 133 |












