Quinolizidine Alkaloids
The lupin alkaloids, found in species of Lupinus (Leguminosae/Fabaceae), and responsible for the toxic properties associated with lupins, are characterized by a quinolizidine skeleton (Figure 25). This bicyclic ring system is closely related to the ornithine-derived pyrrolizidine system, but is formed from two molecules of lysine. Lupinine from Lupinus luteus is a relatively simple structure very comparable to the basic ring system of the pyrrolizidine alkaloid retronecine, but other lupin alkaloids, e.g. lupanine and sparteine (Figure 25) contain a tetracyclic bis-quinolizidine ring system, and are formed by incorporation of a third lysine molecule. Sparteine is also the major alkaloid in broom (Cytisus scoparius; Leguminosae/Fabaceae). The alkaloid cytisine, a toxic component of Laburnum species (Leguminosae/Fabaceae) contains a modified tricyclic ring system, and comparison with the structures of lupanine or sparteine shows its likely relationship by loss of carbon atoms from the tetracyclic system (Figure 25). However, the structural similarity of lupinine and retronecine is not fully reflected in the biosynthetic pathways.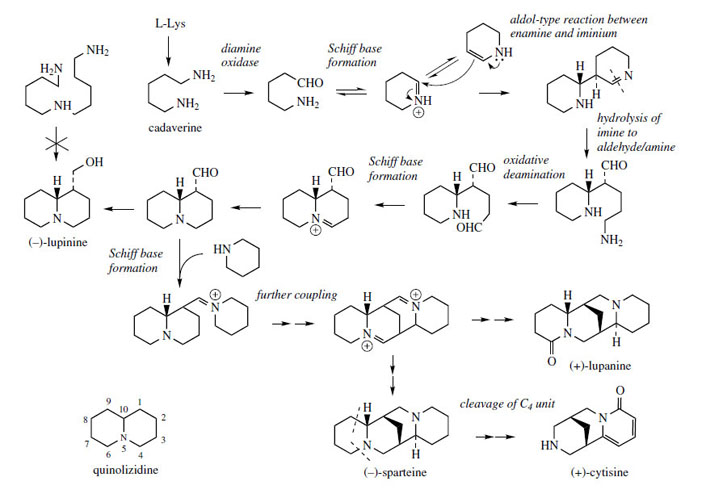 |
| Figure 25 |
Experimental evidence shows lysine to be incorporated into lupinine via cadaverine, but the intermediate corresponding to homospermidine is excluded. Δ1-Piperideine seems to be an important intermediate after cadaverine and the pathway proposed (Figure 25) invokes coupling of two such molecules. The two tautomers of Δ1-piperideine, as N-analogues of corresponding carbonyl compounds, are able to couple by an aldol-type mechanism. Indeed, this coupling occurs in solution at physiological pHs, though stereospecific coupling to the product shown in Figure 25 would require appropriate enzymic participation. Following the coupling, it is suggested that the imine system is hydrolysed, the primary aminem group then oxidized, and formation of the quinolizidine ring is achieved by Schiff base formation. Lupinine is then synthesized by two reductive steps.
The pathway to sparteine and lupanine undoubtedly requires participation of another molecule of cadaverine or Δ1-piperideine. Experimental data are not clear-cut and Figure 25 merely indicates how incorporation of a further piperidine ring might be envisaged. Loss of one or other of the outermost rings and oxidation to a pyridone system offers a potential route to cytisine.
Quinolizidine alkaloids are mainly found in plants of the Leguminosae/Fabaceae family. They deter or repel feeding of herbivores, and are toxic to them by a variety of mechanisms. A number of plants (Laburnum, Cytisus, Lupinus) containing significant quantities of these alkaloids must be regarded as potentially toxic to humans, and are known to be responsible for human poisoning. The widely planted and ornamental laburnum trees offer a particular risk, since all parts, including the pealike seeds, contain dangerously high amounts of alkaloids. So-called ‘sweet lupins’ are selected strains with an acceptably low alkaloid content (typically about a quarter of the total alkaloids of ‘bitter’ strains), which are grown as a high protein crop.




