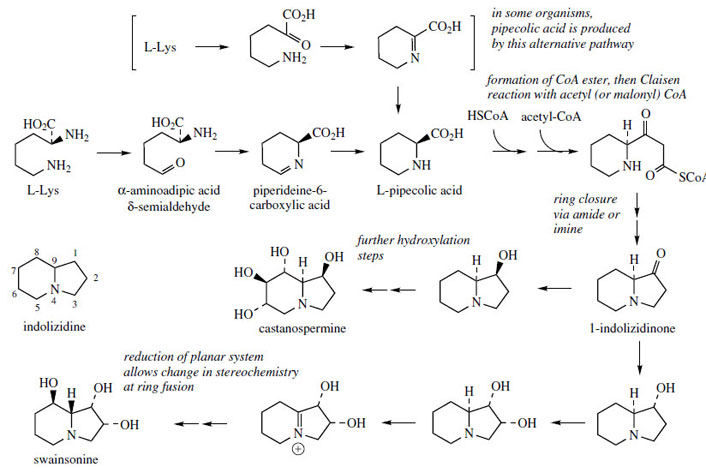
Indolizidine Alkaloids
Indolizidine alkaloids (Figure 26) are characterized by fused six- and five-membered rings, with a nitrogen atom at the ring fusion, e.g.
swainsonine from
Swainsona canescens (Leguminosae/ Fabaceae) and
castanospermine from the Moreton Bay chestnut
Castanospermum australe (Leguminosae/Fabaceae). In this respect, they appear to be a hybrid between the pyrrolizidine and quinolizidine alkaloids described above. Although they are derived from lysine, their origin deviates from the more common lysine-derived structures in that
L-pipecolic acid is an intermediate in the pathway. Two routes to pipecolic acid are known in nature as indicated in Figure 26, and these differ with respect to whether the nitrogen atom originates from the α- or the ε-amino group of lysine. For indolizidine alkaloid biosynthesis, pipecolic acid is formed via the aldehyde and Schiff base with retention of the α-amino group nitrogen.
 |
| Figure 26 |
 |
| Figure 27 |
The indolizidinone may then be produced by incorporating a C
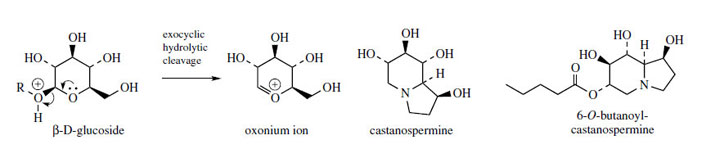
2 acetate unit by simple reactions, though no details are known. This compound leads to castanospermine by a sequence of hydroxylations, but is also a branchpoint compound to alkaloids such as swainsonine, which have the opposite configuration at the ring fusion. Involvement of a planar iminium ion could account for the change in stereochemistry. Polyhydroxyindolizidines such as swainsonine and castanospermine have demonstrated activity against the AIDS virus HIV, by their ability to inhibit glycosidase enzymes involved in glycoprotein biosynthesis. The glycoprotein coating is essential for the proliferation of the AIDS virus. This has stimulated considerable research on related structures and their mode of action. The ester 6-O-butanoylcastanospermine (Figure 27) is currently in clinical trials as an anti-AIDS agent. There is a strong similarity between castanospermine and the oxonium ion formed by hydrolytic cleavage of a glucoside (Figure 27), but there appears to be little stereochemical relationship with some other sugars, whose hydrolytic enzymes are also strongly inhibited. These alkaloids are also toxic to animals, causing severe gastro-intestinal upset and malnutrition by severely affecting intestinal hydrolases. Indolizidine alkaloids are found in many plants in the Leguminosae/Fabaceae (e.g.
Swainsona, Astragalus, Oxytropis) and also in some fungi (e.g.
Rhizoctonia leguminicola).






