Imidazole Alkaloids
The amino acid L-histidine (Figure 110) contains
an imidazole ring, and is thus the likely presursor of alkaloids containing this ring system.
There are relatively few examples, however, and
definite evidence linking them to histidine is often
lacking. Histamine (Figure 110) is the decarboxylation product from histidine and is often involved in human allergic responses, e.g. to insect bites or pollens. Stress stimulates the action of the enzyme histidine decarboxylase and histamine is released from mast cells (Figure 110). Topical antihistamine creams are valuable for pain relief, and oral antihistamines are widely prescribed for nasal allergies such as hay-fever. Major effects of histamine include dilation of blood vessels, inflammation and swelling of tissues, and narrowing of airways. In serious cases, life-threatening anaphylactic shock may occur, caused by a dramatic fall in in blood pressure. |
 |
| Figure 111 |
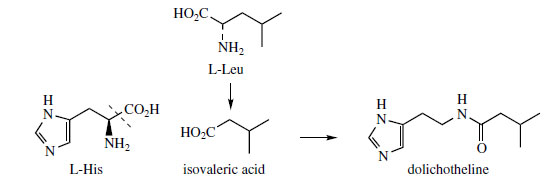
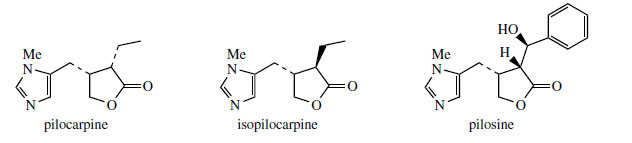
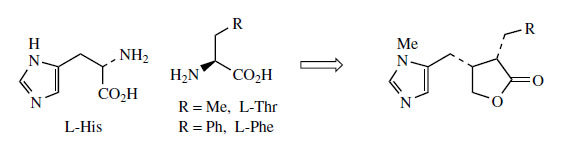
Histidine is a proven precursor of dolichotheline(Figure 111) in Dolichothele sphaerica(Cactaceae), the remaining carbon atoms originating from leucine via isovaleric acid. The imidazole alkaloids found in Jaborandi leaves (Pilocarpus microphyllus and P. jaborandi; Rutaceae)* are also probably derived from histidine, but experimental data are lacking. Jaborandi leaves contain primarily pilocarpine and pilosine (Figure 112). Pilocarpine is valuable in ophthalmic work as a miotic and as a treatment for glaucoma. Additional carbon atoms may originate from acetate or perhaps the amino acid threonine in the case of pilocarpine, whilst pilosine incorporates a phenylpropane C6C3 unit (Figure 113).
 |
| Figure 112 |
 |
| Figure 113 |
Pilocarpus
Pilocarpus or jaborandi consists of the dried leaflets of Pilocarpus jaborandi, P. microphyllus, or P. pennatifolius (Rutaceae), small shrubs from Brazil and Paraguay. Pilocarpus microphyllus is currently the main source. The alkaloid content (0.5–1.0%) consists principally of the imidazole alkaloid pilocarpine (Figure 112), together with small amounts of pilosine (Figure 112) and related structures. Isomers such as isopilocarpine (Figure 112) and isopilosine are readily formed if base or heat is applied during extraction of the alkaloids. This is a result of enolization in the lactone ring, followed by adoption of the more favourable trans configuration rather than the natural cis. However, the iso alkaloids lack biological activity. The alkaloid content of the leaf rapidly deteriorates on storage.
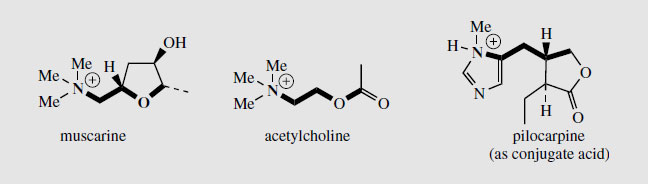
Pilocarpine salts are valuable in ophthalmic practice and are used in eyedrops as miotics and for the treatment of glaucoma. Pilocarpine is a cholinergic agent and stimulates the muscarinic receptors in the eye, causing constriction of the pupil and enhancement of outflow of aqueous humour. The structural resemblance to muscarine and acetylcholine is shown in Figure 114. Pilocarpine gives relief for both narrow angle and wide angle glaucoma. However, the ocular bioavailability of pilocarpine is low, and it is rapidly eliminated, thus resulting in a rather short duration of action. The effects are similar to those of physostigmine and the two agents are sometimes combined. Pilocarpine is antagonistic to atropine. It has been found that pilocarpine gives relief for dryness of the mouth that results in patients undergoing radiotherapy for mouth and throat cancers. As muscarinic agonists, pilocarpine and analogues are also being investigated for potential treatment of Alzheimer’s disease.
 |
| Figure 114 |




