Distillation
Distillation is used to separate the components of a liquid mixture by vaporizing the liquids, condensing the vapours and collecting the liquid condensate. Separation is the result of the differing boiling points of the individual constituents of the mixture and the efficiency of the distillation column. You may be required to purify a liquid by distillation, which involves the removal of small quantities of impurities, or to separate a mixture of liquids by factional distillation, each of which can then be purified by distillation.You will meet several types of distillation process each applicable to different situations depending on the chemicals to be purified or separated.
- Simple distillation: used for separating liquids, boiling below 200°C at atmospheric pressure, from other compounds. For effective separation there should be a difference in the boiling points of the components of at least 80°C.
- Fractional distillation: used for separating components of liquid mixtures, which have boiling points differing by more than 25°C, at temperatures below 200°C.
- Vacuum or reduced-pressure distillation: used for separating liquids boiling above 200°C, when decomposition may occur at the high temperature. The effect of distilling at reduced pressure is to lower the boiling point of a liquid. This technique can be applied to both simple distillation and fractional distillation.
- Steam distillation: used for separating mixtures of chemicals such as oils, resins, hydrocarbons, etc., which are essentially insoluble in water and may decompose at their boiling points. Heating the compounds with steam makes them distil below 100°C.
Distillation equipment
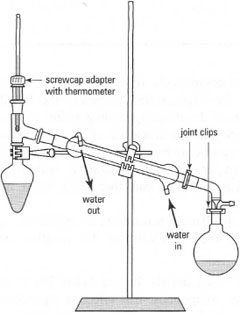
Apparatus used for the various types of distillation has several general features:
- Distillation flask: usually round bottom or pear shaped with one or two necks (to allow a vacuum bleed to be fitted).
- Still-head: to hold the thermometer and to channel the vapour into the condenser. For fractional distillation, the fractionating column is fitted between the distillation flask and the still-head.
- Condenser: usually with circulating cold water.
- Take-off adapter: to allow the distillate to run into the collecting vessel. For vacuum distillation, the adapter will have a vacuum inlet tube and could have three 'arms' to allow three fractions to be collected without breaking the vacuum.
- Receiving (collection) vessel: this can be a test tube, a measuring cylinder, a conical flask, or a round-bottom flask with a ground-glass joint.
*Note: You must ensure that all the ground-glass joints are seated properly and the apparatus is clamped firmly so that no movement of the joints will allow vapours to escape.
 |
| Fig. 15.1 Apparatus for simple distillation. |




