Liquid-liquid extraction
Several experimental processes in practical chemistry are based on liquidliquid extraction:- 'Extraction': where a solid or liquid suspended or dissolved in one solvent is extracted into another. This technique can be used to separate covalent molecules from ionic compounds in an aqueous solution or suspension.
- 'Washing': where ionic species are removed from a non-polar solvent by extraction into water.
- 'Acid-base extraction': where covalent molecules are converted into their salts and thus removed from a non-polar solvent into water, while neutral covalent species will remain in the non-polar solvent, as shown in Table 14.1.
For liquid-liquid extraction, water is usually the polar solvent. Since most extractions involve getting the required compound into the organic solvent (or removing unwanted ionic chemicals from it), it should have good solvent power for the desired compound and a low boiling point for ease of removal and recovery of the compound. The common organic solvents used in liquid-liquid extraction are diethyl ether (ethoxyethane) b.pt. 34°C, dichloromethane (DCM) b.pt. 41°C and ethyl acetate (ethyl ethanoate) b.pt. 77°C. Dichloromethane is denser than water and forms the lower layer, whereas diethyl ether and ethyl acetate float on water and are the upper layer.
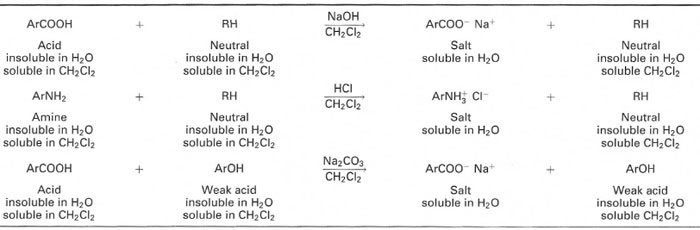 |
| Table 14.1 Examples of acid-base extraction chemistry |
Partition coefficients
The theory of liquid-liquid extraction is based on the equilibrium between the concentrations of dissolved component in the two immiscible liquids, when they are in contact. The equilibrium constant for this process is called the partition coefficient or distribution coefficient and is given by:
⇒Equation [14.1]
| K = | concentration of solute in liquid 1 |
| concentration of solute in liquid 2 |
You only need to calculate such quantities if:
- you are carrying out specific experiments to determine partition coefficients, when you will be given specific instructions or references to the appropriate literature;
- the solute has appreciable solubility in both solvents.
⇒Equation [14.2]
| wn = | w0 | ( | Kv | ) | n |
| Kv + s |
where K = partition coefficient of the solute, v = volume (mL) of aqueous solution of the solute, s = volume (mL) of immiscible organic solvent, w0 = weight of solute initially in the aqueous layer, wn = weight of solute remaining in the aqueous layer after n extractions, and n = number of extractions. Evaluation of this expression shows that, for a fixed volume of solvent, it is more efficient to carry out many small extractions than one big one.
Separatory funnels
These come in a range of sizes from 5mL to 5000mL and there are two general types: parallel sided and cone shaped (Fig. 14.1). Cone-shaped separatory funnels are made of thin glass and should be supported in a ring. Small-volume cone-shaped funnels (< 100mL capacity) and parallel-sided separatory funnels should be clamped at the ground-glass joint at the neck.
Separatory funnels will have glass or Teflon® taps with a rubber ring and clip or screw cap on the end to prevent the tap slipping from the barrel, or a Rotaflo® tap. You must ensure that the tap assembly is in good condition by making the following checks before starting work:
- For glass taps: disassemble the tap by first removing the clip and ring or cap from the tap (note the order of the component parts for reassembling). Dry the tap and barrel with tissue, add a light smear of grease to the tap (making sure you do not clog the hole in the tap) and reassemble the tap and fittings, turning the tap to ensure free movement. Support the separatory funnel in position and add some of the organic solvent to be used (2-3 mL) to the funnel, with the tap closed, to check that the tap does not leak. If the tap leaks, disassemble and regrease.
- For Teflon® taps: disassemble the tap, wipe the tap and barrel with clean tissue, reassemble without grease, check for free movement of the tap and for leakage as described above. When you have finished using the funnel, loosen the clip/cap on the tap since Teflon® will flow under pressure and the tap may 'seize' in the barrel.
- For a Rotaflo® tap: unscrew the tap from the funnel and ensure that the plastic locking thread is in place (Fig. 14.2). If it is not present, consult your instructor and obtain a replacement. Dry the barrel of the tap and the tap with a tissue and reassemble. Do not grease the Rotaflo® tap.
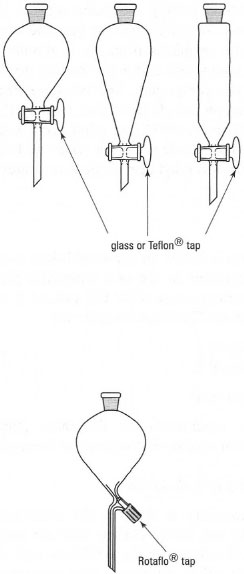 |
| Fig. 14.1 Separatory funnels. |
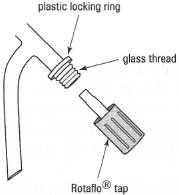 |
| Fig. 14.2 A Rotaflo® tap. |
The general procedure for using a separatory funnel for extraction is described in Box 14.1 and there are five additional practical tips to aid your success:
- Label all flasks to avoid confusion.
- Never throwaway any of the separated liquids until you are absolutely sure of their identity.
- Always transfer solvents into the separatory funnel using a stemmed filter funnel so that solids and liquids will not stick to the inside of the joint and prevent a good seal when you insert the stopper and then invert the funnel.
- Always place a 'safety' beaker under the separatory funnel to collect liquid just in case the tap leaks (Fig. 14.3a).
- Always take the stopper from the separatory funnel before you attempt to allow liquid to run from the funnel. If you do not remove the stopper from the top of the funnel, a vacuum is formed in the funnel after a little of the liquid has run out. Air will be sucked into the funnel through the outlet stem causing bubbles, which will remix your separated layers. If your funnel is equipped with a Quickfit® stopper, it is good practice to take the stopper out of the top and put it back upside down (Fig. l4.3b). This ensures that no vacuum is formed and that organic vapours do not escape easily from the flask.
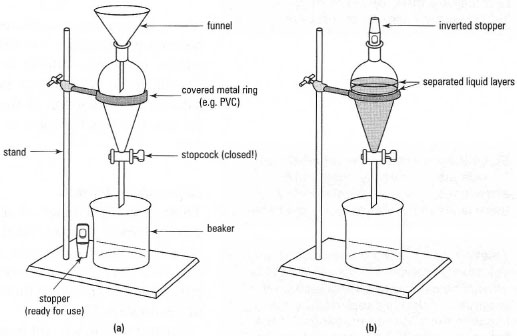 |
| Fig. 14.3 A separatory funnel (a) ready to use and (b) in use. |
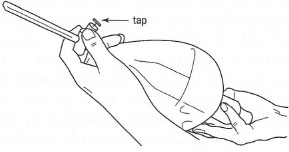 |
| Fig. 14.4 Holding a separatory funnel. |




