The recrystallization process
Having chosen a suitable solvent system, the process to be used to purify the bulk of your impure compound can be separated into several distinct steps:- Dissolution of the solid using a single-solvent or a mixed-solvent system.
- Decolorization using charcoal: even if your compound is white, decolorization will improve its appearance significantly.
- Hot filtration to remove solvent-insoluble impurities and charcoal.
- Cooling, to produce the crystals.
- Collection of the crystals by suction filtration.
- Drying.
Dissolution
To carry out a single-solvent recrystallization (Box 13.3) you must get the compound into solution and this is achieved by suspending it in the appropriate cold solvent, found in the solvent selection process, and then heating the mixture to dissolve the solid. The equipment used will depend on the boiling point of the solvent, its flammability and toxicity. Some general systems are shown in Table 13.2.
When heating solvents in conical flasks and beakers you should cover the top of the flask with a clock-glass or watch-glass to prevent excessive evaporation of the solvent, resulting in the formation of crystals on the sides of the flask above the surface of the solution. Do not forget to take the appropriate 'anti-bumping' precautions, because you may need to boil the mixture for several minutes to achieve complete dissolution of the chemical(s). You should use a glass rod, a wooden 'boiling stick' or antibumping chips in conical flasks of volume up to 500mL; magnetic 'fleas' or anti-bumping granules in round-bottom flasks in reflux apparatus; magnetic 'fleas' or stirring bars in large-volume glassware (> 500 mL).
 |
| Table 13.2 Solvents for recrystallization |
Decolourization
Small amounts of coloured impurities can be removed from your product by absorption on finely divided charcoal, which is then removed in the hot filtration process. In general, you should use charcoal in every recrystallization since the colour of white and coloured compounds is improved by 'decolorization'.
The amount of charcoal used should be about 2% by weight of the sample to be recrystallized. Note the following important points:
- Add the charcoal only when the solution has been formed. If you add charcoal to the cold solution, you will not be able to see when all of the compound has dissolved.
- Do not add charcoal to a boiling or a very hot solution otherwise the solution will boil extremely vigorously, usually boiling out of the flask or reflux apparatus.
- To add charcoal to a hot solution, remove the flask from the heat source and then let it cool a little (not enough to cause precipitation). Add the charcoal either from a spatula (into a conical flask or beaker), a paper funnel or powder funnel (into a round-bottom flask with a ground-glass joint in reflux apparatus), and then reheat the solution.
Hot filtration
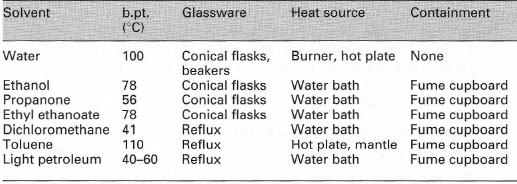
This is a modification of gravity filtration, designed to remove solvent-insoluble impurities, charcoal, anti-bumping granules or magnetic 'fleas' from the hot solution before cooling the solution to form the crystals of purified product.
*Note: Hot filtration is used to prevent cooling of the solution during the filtration process, which would result in the formation of crystals in the filter paper.
For a successful hot filtration the solution must pass through the filter paper and filter funnel into the collection flask as quickly as possible so that cooling and crystallization do not occur. The following points should be noted:
- Always use a fluted filter paper.
- Always use a 'stemless' glass filter funnel because cooling and thus crystallization may occur in the funnel stem causing a blockage.
- Always heat the filter funnel and filter paper, either by pre-heating them in an oven or by using boiling solvent in the collecting flask (Fig. 13.1).
- If filtration is rapid, keep the filter cone 'topped up' with the hot solution being filtered, since this keeps the filter cone and filter funnel hot. If you allow the filter cone to empty of liquid, cooling and crystallization may occur.
- When attempting a hot filtration with a mixed-solvent system, always ensure that the 'ideal' solvent ratio has not been reached, i.e. there is not enough of the poor solvent to cause immediate precipitation as a result of slight cooling during the hot filtration. The solvent ratio can be adjusted to the 'ideal' ratio for maximum recovery, after filtration and before cooling (Box 13.4).
 |
| Fig. 13.1 Filtration of a hot solution. |
Cooling
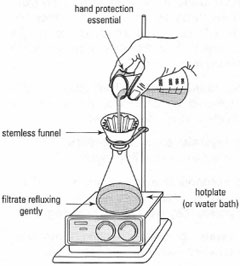
Rapid cooling in an ice-water bath ('crash-crystallization') usually produces small crystals occluded with mother-liquor, whereas slow cooling by allowing the collection flask to stand on the laboratory bench often produces large well-defined crystals. Remember to:
- cover the top of the collection flask with a watch- or clock-glass to prevent solvent evaporation and entry of dust into the flask: do not use rubber bungs or corks since they may be pulled into the flask as it cools;
- clamp the flask in place, if you use an ice-water bath, otherwise it may fall over as the ice melts and the volume of water increases;
- make sure that the solution is cold, even after slow cooling, so that maximum precipitation of the solid occurs.
 |
| Fig. 13.2 'Scratching': (a) right; (b) right; (c) wrong. |
Collection of the crystals
You should collect the purified crystals by suction filtration by the procedure described in Box 5.2. Remember to transfer the crystals from the collecting flask to the filter using a little of the filtrate: do not use fresh solvent. The filtrate is a saturated solution of the compound being recrystallized and cannot dissolve any more solute, but fresh solvent will dissolve some of your product resulting in an inefficient recrystallization process.
Drying
The purified compound should be dried by the appropriate method. If you do not know the melting point of your compound, you should always carry out a test by placing a small amount on a watch-glass in the oven, before committing the bulk of your chemical.
Detailed procedures for single-solvent and mixed-solvent recrystallizations are shown in Box 13.3 and Box 13.4 respectively and the modifications necessary for the use of other solvent systems can be worked out from the information in Table 13.2.




