Evaporation of solvents
There are three commonly used techniques for solvent evaporation:- Evaporation from open flasks on a steam bath.
- Rotary film evaporation.
- Gas 'blow-down'.
- the volume of solvent to be removed;
- the amount of solute in solution;
- the relative boiling points of the solvent and solute;
- the next step in the experimental procedure.
Evaporation from open flasks
This is useful for evaporating small volumes (~ 25mL) of low-bailing-point solvents (< 70°C) from solutions containing a solute which has a boiling point above 110°C.Place the solution in a beaker or conical flask, containing a glass rod or boiling stick, onto a steam bath (maximum temperature achievable is 100°C) in a fume cupboard and evaporate the solvent until boiling ceases. The obvious advantage is simplicity; the disadvantages include environmental concerns of release of solvents in the atmosphere, contamination of the solvent by condensed steam and incomplete solvent removal due to the 'hold-up' volume of the flask.
Rotary film evaporation
This method, which is also known as rotary evaporation or 'rovap', is the technique of choice for the removal of large volumes of volatile solvents from solutions, e.g. from extractions and column chromatography. Rotary evaporators are now standard communal pieces of equipment in the laboratory and the operating principle is that of a reduced-pressure distillation except that the evaporation flask can be rotated. This rotation reduces the risk of 'bumping', inherent in all reduced-pressure distillations, and spreads the solution in a thin film on the walls of the flask. This effectively increases the surface area of the solution and increases the rate of evaporation, which is further enhanced by the use of a vacuum.
There are many variations in the details of the form of rotary film evaporators and a typical assembly is illustrated in Fig. 17.1. A general guide to the use of a rotary evaporator is given in Box 17.1.
*Note: When using a 'rovap' you must check that the reduced- pressure boiling point of the solute you are trying to isolate is below the temperature of the water bath.
When using rotary film evaporators you should take note of the following safety advice:
- Never use flat-bottom flasks or conical flasks under reduced pressure.
- Always check your flask for 'star' -cracks.
- Always make sure that your solution has cooled to room temperature before you begin, otherwise it may boil vigorously and 'bump' when you apply the vacuum, before it is lowered into the water bath.
- Do not rush to lower the flask into the water bath: wait to see what happens to the extent of evaporation at room temperature.
- Always have the water bath just warm, not hot, at the start of the procedure. If the water bath is too hot, allow it to cool or add cold water or Ice.
- Check that all joints are 'sealed' and that the water pump is producing a vacuum: it will change 'note' as the vacuum is produced, when it is working properly. If there is no vacuum, the solution may not boil and you will overheat it in trying to promote evaporation. The joints may suddenly seal and the solution will then boil vigorously under the reduced pressure and will 'bump' into the condenser and receiving flask.
If it is necessary to evaporate volumes of solvent greater than the capacity of the rotating flask, you can carry out the process batch-wise involving several separate evaporations or the rotary evaporator can be modified for continuous evaporation (Fig. 17.2). A thin Teflon® tube is attached to the vacuum inlet adapter so that it feeds down the condenser into the 'barrel' and another glass tube, dipping into the solution to be evaporated, is connected to the air inlet on the vacuum adapter. Once the rotary evaporator is operating, the tap on the vacuum adapter is opened a little. Solution is drawn up by the vacuum, runs into the rotating flask and is evaporated. Careful control of the tap allows a constant volume of solution to be sucked into the rotating flask and evaporated without overfilling it.
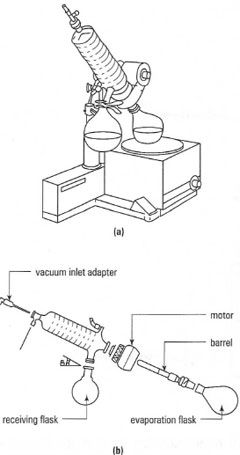 |
| Fig. 17.1 (a) Typical examples of a rotary evaporator; (b) exploded view of glassware. |
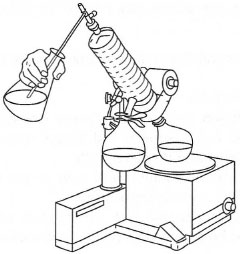 |
| Fig. 17.2 The procedure for continuous solvent removal using a rotary evaporator. |
Gas 'blow-down'
This procedure is useful for removing very small volumes of solvents (about 2 mL) from solutes by blowing a stream of nitrogen over the surface of the solution, while warming the solution gently. The main application of the gas blow-down is in the isolation of small amounts of solute from rotary evaporation or small-scale liquid-liquid extraction, for further analysis by instrumental techniques, where the sample size may be 20 mg or less: for example, infrared spectroscopy, NMR spectroscopy, gas chromatography or liquid chromatography.
The simplest system for evaporation by gas blow-down is shown in Fig. 17.3. A Pasteur pipette is connected by a flexible tube to a cylinder of nitrogen, which has a gas blow-off safety system. The sample is placed in a special tube with a conical base, such as a ReactiVial®. Hold the Pasteur pipette and direct a gentle stream of nitrogen towards the side of the tube so that it flows over the surface of the liquid. As the solvent evaporates, the liquid and tube will cool and may condense atmospheric water into the tube. To prevent condensation, clamp the tube in a warm sand bath or above a closed steam bath or in the hole of a purpose-designed aluminium heatingblock, The following points should be noted when using a gas blow-down system:
- Always carry out the operation in a fume cupboard.
- The solute should have negligible vapour pressure at room temperature, otherwise it may eo-evaporate with the solvent.
- Do not heat the solution to boiling. Only apply enough heat to prevent condensation of atmospheric water vapour.
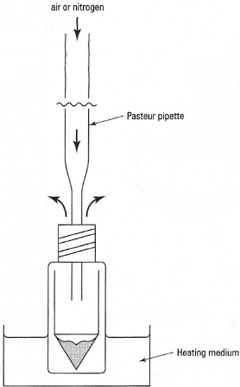 |
| Fig. 17.3 Gas 'blow-down' evaporation. |




