Reflux
Reflux is one of the most common techniques you will encounter in your chemistry laboratory classes. Since many reactions between covalent compounds are slow processes rather than instantaneous reactions, prolonged heating forces the equilibrium to give an acceptable amount of product. In the reflux process, the reactants are dissolved or suspended in a suitable solvent, the solvent is boiled and then condensed so that it returns to the reaction flask. Once set up, a reaction carried out under reflux can be run for minutes, hours or even days to promote the required reaction. The basic components for a reflux apparatus are:- a reaction flask;
- a reflux condenser;
- a heat source;
- a coolant source, usually water, for the condenser.
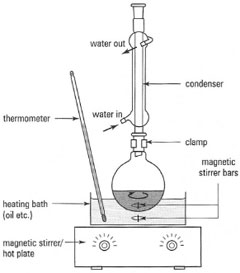 |
| Fig. 16.1 Apparatus for simple reflux. |
Condensers
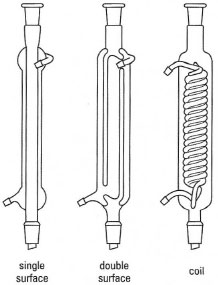
For general-purpose work, these are usually single-surface or double-surface types (Fig. 16.2): the double-surface condenser is used for low-boiling point solvents such as dichloromethane, diethyl ether or light petroleum (b.pt. 40- 60°C).
Rubber tubing for coolant water is attached to the condenser in two ways:
- If the condenser has glass inlet and outlet pipes with a 'knuckle', wet the inside of the rubber tube with a little water and slide it onto the pipe and past the 'knuckle'. The rubber tubing must be a tight fit otherwise it may slide off over a period of time.
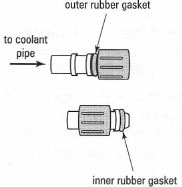
- Modern condensers have plastic adapters, which can be attached to the
tubing and then screwed on the threaded inlet and outlet pipes. Slide the
rubber tubing onto the moistened 'pipe' on the adapter, and then screw
the adapter onto the condenser. You must ensure that the adapter has a
rubber gasket on the inside of the threaded portion (Fig. 16.3), otherwise
it will leak water at the condenser inlet or outlet.
*Note: You should always attach rubber tubing to a condenser before fitting it to the apparatus.
|
|
Drying tubes
Water can get into your reaction by condensation from the atmosphere or by condensation of the steam produced in a water bath. To exclude water, you should fit a guard tube containing a solid drying agent such as anhydrous calcium chloride or calcium sulphate to the top of the condenser. A typical guard tube is shown in Fig. 16.4; use a coarse-sized drying agent rather than a fine powder, which would 'cake' very quickly as it absorbs moisture.
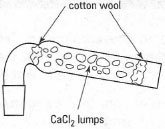 |
| Fig. 16.4 A CaCI2 guard tube. |
Reflux with addition of chemicals
Instead of stopping the reaction and opening the apparatus, you can put in the new chemicals using an addition or 'dropping' funnel. Addition funnels are separatory funnels with a ground-glass joint on the stem, and there are two types (Fig. 16.5):
- Addition funnels: when you add a liquid or solution from the funnel to the reaction flask, you must take out the stopper, otherwise a vacuum is formed and the liquid does not flow out. This is a disadvantage when using compounds with irritating or toxic vapours or which are air sensitive. The simplest solution to this problem is to fit a guard tube, instead of a stopper, to the addition funnel and then the liquid or solution will flow easily into the reaction flask.
- Pressure-equalizing dropping funnels: these have a side arm linking the reservoir of the funnel to the inlet stem below the tap. The pressures in the reservoir and the reaction flask are equal, and liquid will flow into the reaction flask even when the stopper is in place in the funnel. Pressure-equalizing dropping funnels are very expensive and are normally only used for inert atmosphere reactions.
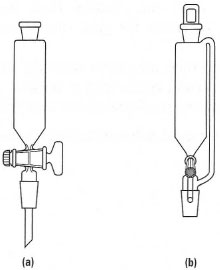 |
| Fig. 16.5 Adding chemicals to a reflux apparatus: (a) addition funnel; (b) pressureequalizing funnel. |
Reflux with mechanical stirring
When a magnetic stirrer is unsuitable, e.g. in reactions involving viscous liquids or mixtures of solids and liquids, a mechanical stirrer must be used. A mechanical or overhead stirrer system comprises:
- An electric variable speed motor connected to the mains supply.
- A flexible connector, usually a short length of rubber tubing.
- A stirrer gland, which fits into the top ground-glass joint of the flask acting as a seal for the refluxing vapour and a guide for the stirrer shaft. There are several types of gland available, but the best is now made from Teflon® and needs no lubrication. If this is not available, a screwcap adapter in which the rubber sealing ring has been lubricated with a touch of silicone oil can be used. Note that the stirrer shaft should rotate in the adapter, not the adapter in the joint of the flask, so do not tighten the screwcap too much.
- A Teflon® paddle which swivels on the end of the stirrer shaft so that it can pass through the ground-glass joint on the top of the flask.
- The weight of the stirrer motor high up on the support stand: use a large support stand with a heavy base or use a support framework, which is screwed to the laboratory bench. Besides the motor's weight, the torque of the motor can cause 'whipping' in the support stand.
- The motor, stirrer gland, stirrer shaft and reaction flask must be absolutely vertical and concentric, otherwise the glass stirrer shaft will snap.
- Since there will always be a little sideways movement when the stirrer is operating, the flask and the motor should be clamped in position on the same stand. Condensers and addition funnels should not be clamped.
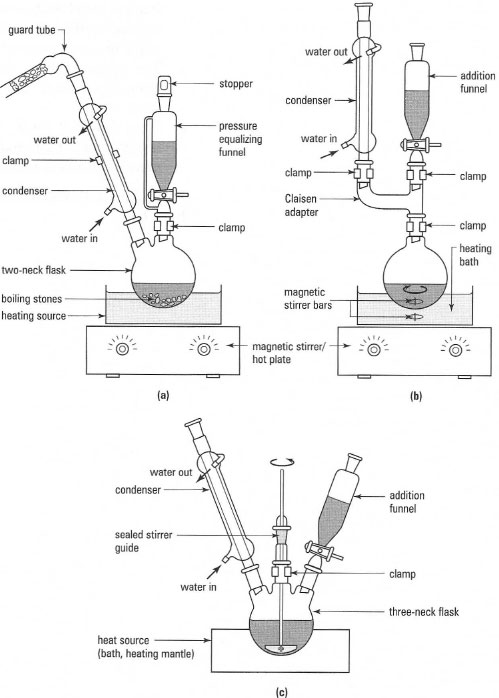 |
| Fig. 16.6 Apparatus arrangements for reflux with addition: (a) heating a reaction mixture to reflux during the addition of a reagent; (b) heating a stirred reaction mixture during the addition of a reagent; (c) heating and overhead stirring of a reaction mixture during the addition of a reagent. |