Fractional distillation
Simple distillation is not very effective in separating liquids unless their boiling points are at least 80°Capart and a better separation can be achieved if a fractionating column is used. There are many types available (Fig. 15.2) and the device brings the ascending vapours into contact with the condensing (in the fractionating column) liquid and amounts to a succession of many simple distillations in which the descending liquid strips the high-boiling component from the ascending vapour. Overall, the lower boiling component distils first and cleanly. The column packing, usually glass beads, helices or 'fingers', gives a large surface area for contact of the ascending vapours and descending liquid. After the first fraction has distilled, the temperature will rise and the rate of distillation will slow. This is an intermediate fraction containing a little of both components of the mixture. Finally, the temperature will become constant and the pure higher boiling compound will distil. The procedure for fractional distillation is given in Box 15.3.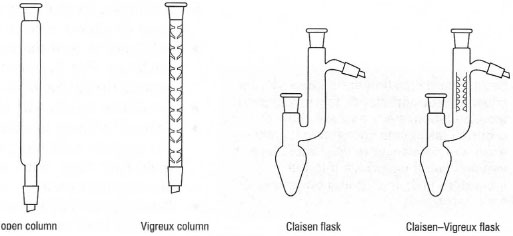 |
| Fig. 15.2 Common fractionating columns. |
*Note: A successful fractional distillation relies on thermal equilibration of the components in the fractionating column. You must allow the column to be heated by the vapours of the mixture.




