Inert atmosphere
The source of the inert atmosphere is usually a cylinder of nitrogen or argon gas under pressure, which should be placed as close to the apparatus as possible to avoid long 'runs' of connecting rubber tubing.Gas cylinders
The gas flow rate from the cylinder is controlled by the cylinder head regulating valve (Fig. 18.1). Before you start make sure that the regulator outlet tap is off (turn anti-clockwise until it feels 'free') and then open the valve to the cylinder with the cylinder spanner (turn anti-clockwise) and the cylinder pressure should be indicated on the right-hand pressure dial. Switch on the gas at the regulator (turn slowly clockwise) until there is a reading on the left-hand dial. Use the minimum pressure required to provide a steady flow of gas. The gas flow rate from the regulator can be controlled further by a needle valve on the regulator outlet, if one is fitted. To switch off, reverse the instructions above.
Connection to the apparatus
Use clean, dry, thin-walled rubber tubing and special adapters with groundglass joints to connect the tubing to your reaction flask or to the inlet pipe of a 'bubbler'. Where a single cylinder supplies several outlets, e.g. in a fume cupboard, the gas flow rate may change markedly when someone turns off one of the outlets, resulting in an increase in gas pressure to your equipment. You should, therefore, fit a gas 'blow-off valve between your gas supply and the apparatus.
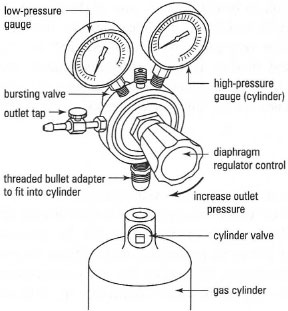 |
| Fig. 18.1 The diaphragm pressure regulator. |
Gas 'bubblers'
The exit of the inert gas from the apparatus must be protected by a gas 'bubbler'. The 'bubbler' allows you to monitor the flow of inert gas through the apparatus and prevents the entry of air into the apparatus. Several designs of gas 'bubbler' are available (Fig. 18.2) and it is usual to connect the 'bubbler' to the apparatus at the top of the condenser. You should make sure that the 'bubbler' contains enough mineral oil to create a seal from the atmosphere and that it has a bulb above the mineral oil to collect any mineral oil, which could be sucked back into your apparatus if there is a sudden contraction in the volume of inert gas in the apparatus. Such changes in volume will occur if you suddenly cool the apparatus without increasing the gas flow to compensate for the resulting reduction in inert atmosphere volume.
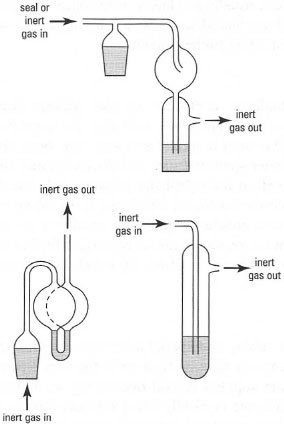 |
| Fig. 18.2 Typical 'bubblers'. |




