Recrystallization
The products from many synthetic preparations are seldom pure and the technique of recrystallization, which involves dissolving the impure material in a hot solvent and then cooling the solution to produce crystals, is routinely used to purify covalent organic and inorganic solids.In general there are three types of impurities, which are removed by the recrystallization process:
- Insoluble material: anti-bumping granules, pieces of filter paper, traces of drying agents, grit, hair and other materials which may have been present in the starting chemicals.
- Small quantities of unreacted starting chemicals and/or by-products from side reactions or other isomers.
- Very small amounts of coloured by-products resulting from oxidation or polymerization of the chemicals used.
When the crude reaction product is dissolved in the hot solvent the insoluble impurities (type 1 above) can be removed by hot filtration. When the hot solution is allowed to cool, the solution becomes saturated with the desired compound and it precipitates from the cold solution. The cold solution does not become saturated with the lower concentration of the contaminants of type 2, which therefore remain in solution. Coloured impurities (type 3) can be removed by absorption using charcoal. The recrystallization process can be divided into three separate steps:
- Selection of a suitable solvent.
- Recrystallization of the crude compound.
- Drying the purified solid.
Solvents for recrystallization
You can decide on a suitable solvent for recrystallization of your crude chemical product in several ways:
- The experimental protocol may tell you which solvent to use.
- If you know the identity of the compound you have made, reference texts may indicate a suitable solvent).
- If you are not sure of the identity of the compound you have prepared or if it is a new compound, you must carry out a 'solvent selection' to find out which solvent is the most appropriate.
When you are choosing a solvent for recrystallization, look for the following general characteristics:
- A high dissolving power for the solute at high temperature and a low dissolving power at room temperature or below, so that a high recovery of purified compound can be achieved.
- A high or negligible dissolving power for the impurities, so that they will either be filtered off or remain in solution.
- A relatively low boiling point, to facilitate drying the purified compound.
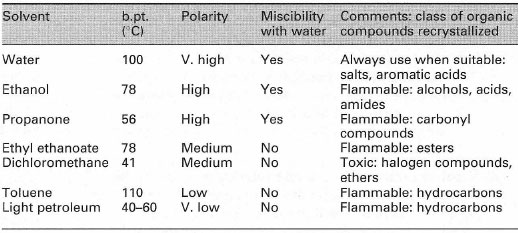 |
| Table 13.1 Selected solvent properties |
Solvent selection
To find a suitable solvent for recrystallization you must carry out a series of tests measuring the solubility of your crude compound in a series of solvents (cold and hot) of varying solvent polarity. This series of tests is called 'solvent selection' and is carried out on a test tube scale but, as your technique improves, you can carry out the tests using semi-micro scale since you will use less of your compound during the process. The procedure for solvent selection at the test tube scale is described in Box 13.1 and modification of the procedure to semi-micro scale requires only a corresponding reduction of the quantities of solvent and solute used.
*Note: When carrying out the solvent selection experiments you must always cool the solution after heating. Do not assume that if the compound is insoluble in the cold solvent and dissolves when heated, it will always precipitate on cooling.
Mixed solvents
When no single solvent is found to be suitable for recrystallization, then a mixed solvent system must be used. There are three essential properties required for a pair of solvents to be used in a mixed-solvent system:
- The two solvents must be miscible in all proportions over the temperature range to be used.
- The solute must be insoluble in one of the solvents.
- The solute must be soluble in the other solvent.
Suitable common solvent pairs from the solvents given in Table 13.1 are water/ethanol and dichloromethane/light petroleum (b.pt. 40-60°C), but many other combinations are possible. One of the most frequently encountered mixed solvent systems is 'aqueous ethanol' (water/ethanol) in which the compound to be recrystallized is insoluble in water and very soluble in ethanol.
You can identify the solubility characteristics of your compound using the solvent selection procedure shown in Box 13.1 and modifications for mixed solvent selection are given in Box 13.2.




