Thermometer calibration
Your thermometer may be a major source of error in melting point measurement. Occasionally thermometers for routine laboratory use may not be accurate and may read up to 10°C high or low. To avoid this problem you should always calibrate your thermometer to determine any error and be able to correct for it.To calibrate your thermometer you must measure the melting points of a series of very pure compounds, available commercially, having a range of melting points similar to the range over which you will use the thermometer; a general-purpose series is shown in Table 12.1. Having measured the melting points of each of the pure compounds, take the mid-point of each value for the thermometer reading of melting point for each compound and the midpoint of the literature melting point of each compound and plot them graphically using literature temperature as the y-axis and the thermometer reading as the x-axis, The straight-line plot will obey the equation y = mx + c, where y = real temperature (literature)
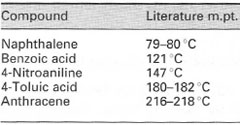 |
| Table 12.1 A typical series of standards for thermometer calibration |




