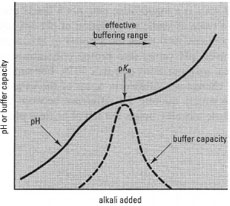
Buffer capacity and the effects of pH
The extent of resistance to pH change is called the buffer capacity of a
solution. The buffer capacity is measured experimentally at a particular pH
by titration against a strong acid or alkali: the resultant curve will be strongly
sigmoidal, with a plateau where the buffer capacity is greatest (Fig. 7.1). The
mid-point of the plateau represents the pH where equal quantities of acid and
conjugate base are present, and is given the symbol pKa, which refers to the
negative logarithm (to the base 10) of the acid dissociation constant, Ka,
where, ⇒ Equation [7.6]
By rearranging eqn [7.6] and taking negative logarithms, we obtain: ⇒ Equation [7.7] |
|
| pH = | pKa + log10 | [A−] |
| [HA] |
This relationship is known as the Henderson-Hasselbalch equation and it shows that the pH will be equal to the pKa when the ratio of conjugate base to acid is unity, since the final term will be zero. Consequently, the pKa of a buffer solution is an important factor in determining the buffer capacity at a particular pH. In practical terms, this means that a buffer solution will work most effectively at pH values about one unit either side of the pKa.
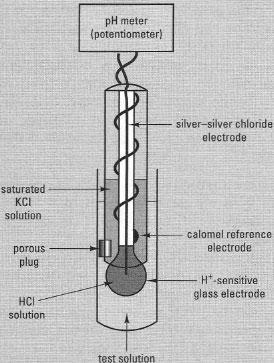 |
| Fig 7.2 Measurement of pH using a combination pH electrode and meter. The electrical potential difference recorded by the potentiometer is directly proportional to the pH of the test solution. |