Preparing solutions
The steps involved in making up general-purpose aqueous solutions are:- Find out or decide the concentration of chemical required and the degree of purity necessary
- Decide on the volume of solution required
- Find out the relative molecular mass of the chemical (Mr). This is the sum of the atomic (elemental) masses of the component element(s)nd can usually be found on the container. If the chemical is hydrated, i.e. has water molecules assosiated with it, these must be included when calculating the mass required.
- Work out the mass of chemical that will give you the concentration desired in the volume required, bearing in
mind the quoted percentage purity of the chemical.
Example 1: Suppose your procedure requires you to prepare 250mL of 0.1 mol L-1 sodium chloride solution- Begin by expressing all volumes in the same units, either millilitres or litres (eg. 250 mL as 0.25 L)
- Calculate the number of moles required from eqn [4.1]:01 = amount
(mol) ÷ 0.25.
By rearrangement, the required number of moles is thus 0.1 × 0.25 = 0.025 mol. - Convert from mol to g by multiplying by the relative molecular mass (M r for NaCl = 58.44g mol-1).
- Therefore, you need to make up 0.025 × 58.44 = 1.46g up to 250 mL of solution, using distilled water
Example 2 : Suppose you are required to make up 100 mL of sodium carbonate (2M).- Convert 2M into mol L-1; concentration required = 2 mol L-1.
- Express all volumes in the same units: therefore 100 mL = 0.1 L.
- Calculate the number of moles required from eqn [4.1]:2 = amount (mol) ÷ 0.1. By rearrangement, the required number of moles is thus 2 × 0.1 = 0.2 mol.
- Convert from mol to g by multiplying by the Mr but note from the container that the compound is Na2CO3.10H2O. Therefore the Mr required must include the water of crystallization and Mr = 286.14g mol-1
- Therefore, you need to make up 0.2 × 286.14 = 57.2g up to 100mL of solution using distilled water.
- Weigh out the required mass of chemical to an appropriate
accuracy. If the mass is too small to weigh with the desired degree
of accuracy, consider the following options:
- Make up a grater volume of solution.
- Make up a more concentrated solution, which can be diluted at a later stage.
- Weigh the mass first, and calculate what volume to make the solution up to afterwards using eqn 4.1.
- Add the chemical to a beaker or conical flask and than the final volume required. If some of the chemical sticks to the weighing receptacle, use some of the water to wash it off. For accurate solutions, for accurate weighing and quantitative transfer.
- Stir and, if necessary, heat the solution to ensure all the chemical dissolves. You can determine visually when this has happened by observing the disappearance of the crystals or powder. Allow the solution to cool, if heated.
- Make up the solutions to the desired volume. If the concentration
needs to be accurate, use a volumetric flask; if a high degree of
accuracy is not required, use a measuring cylinder.
- Pour the solution from the beaker into the measuring vessel using a funnel to avoid spillage, using water to rinse out the vessel.
- Make up the volume so that the meniscus comes up tp the appropriate measurement line. If accuracy is not a major concern, the graduation marks on the beaker or conical flask may be used to establish the approximate volume.
- Transfer the solution to a reagent bottle or conical flask and label the vessel clearly, including hazrd information, where appropriate. Do not use water in this final transfer since you will alter the concentration of the solution by dilution.
The concentration you require is likely to be defined by the protocol you are following and the grade of chemical and supplier may also be specified. To avoid waste, think carefully about the volume of solution you require, though it is always advisable to err on the high side because you may spill some, make a mistake when dispensing or need to repeat part of the experiment. Choose one of the standard volumes for vessels, as this will make measuring out easier.
Use distilled or deionized water to make up aqueous solutions and stir with a clean Pyrex® glass rod or magnetic stirrer bar ('flea') until all the chemical is dissolved. Magnetic stirrers are a convenient means of stirring solutions but precautions should be taken to prevent losses by splashing. Add the flea to the empty beaker or conical flask, add the chemical and then some water. Place the vessel centrally on the stirrer plate, switch on the stirrer and gradually increase the speed of stirring. When the crystals or powder have dissolved, switch off the stirrer and remove the flea with a magnet. Take care not to contaminate your solution when you do this and rinse the flea into the solution with distilled water. In general it is convenient to use glass rods with beakers - ease of access for stirring - and magnetic fleas with conical flasks - lower losses through splashing - but often it is a matter of your preference and laboratory skills.
'Obstinate' solutions may require heating, but only do this if you know that the chemical will not be damaged at the temperature used. Use a stirrer-heater to keep the solution mixed as you heat it. Allow the solution to cool to room temperature before you finalize its volume.
The key features in the preparation of solutions for analytical purposes are:
- Make sure that you have the most accurate available knowledge of masses of the chemicals used.
- Ensure that you have the most accurate available knowledge of the volumes of solutions used.
'Prepare a standard solution (250.00 mL) of ammonium ferrous sulphate (approximately 0.1 M), which is to be used to determine the concentration of a solution of potassium permanganate by titration'.
You must be aware of the following embedded information:
- This is a quantitative experiment, therefore requiring an analytical solution to be prepared.
- You must use a 250.00 mL volumetric flask, which you should note is calibrated at 20°C.
- You must weigh accurately, to four decimal places, the mass of the chemical.
- It is almost impossible to weigh the exact mass of chemical for a specific concentration. For example, the mass of (NH4)2FeS04.6H20 required to prepare 250.00mL of 0.1 M solution is 9.8035g and you cannot weigh out this exact mass. However, you can weigh out a known mass to four decimal places accurately. From this you can then calculate the exact concentration of the chemical in solution, since you will know both mass and volume to a high degree of accuracy.
The main practical point is that you must not lose, by splashing or failure to
transfer by inadequate rinsing, any of the solution being prepared in the
beaker and you must transfer all of the solution, by repeated rinsing, into the
volumetric flask. Therefore it is good practice to use only a glass rod to stir
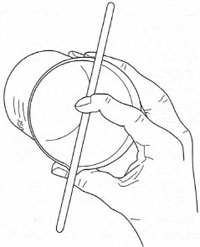
the solution gently to dissolve the solid and to use the glass rod, as shown in
Fig. 4.1, to pour the solution into the filter funnel. This technique, with
practice, prevents losses of solution down the side of the beaker via the
spout; rinsing with water can be achieved by use of a wash bottle squirted
directly into the beaker. You should not use a flea to stir a solution in the preparation of a standard solution, since this introduces more washing steps - washing the flea and the 'flea extractor' - and you still need to use the glass rod for quantitative transfer. Procedures for the method for the preparation of the standard solution are given below. Procedure: How to make an aqueous solution of known concentration from a solid chemical for use in quantitative analysis. |
|
Example: Suppose you are to prepare a standard solution (250.00 mL) of ammonium ferrous sulphate (approximately 0.1 M), which is to be used to determine the concentration of a solution of potassium permanganate.
- This is a quantitative experiment so the ammonium ferrous sulphate must be of the highest purity available to you
- Work out the mass of chemical that will give you the concentrtaion desired in the volume required.
- Convert 1.0M into mol L-1; concentration required = 1.0,ol L-1
- Express all volumes in the same units; therefore 250.00mL = 0.25L
- Convert the number of moles required from eqn [4.1]:1.0 = amount (mol) ÷ 0.25. By rearrangement; the required number of moles is thus 1.0 × 0.25 = 0.025mol.
- Convert from mol to g by multiplying by the Mr, but note from the container that the compound is (NH4)2FeS04.6H20. Therefore the Mr required must include the waterof crystallization and Mr = 392.14g mol-1
- Therefore, you need to weigh out 0.025 × 392.14 = 9.8035g of (NH4)2FeS04.6H20.
- Place a clean, dry weighing boat or approximately sized samle tube onto a simple two-decimal-place balance and zero (tare) the balance and weigh about 9.80g of the chemical.
- Carefully transfer the sample tube plus chemical to a four- decimal-place analytical balnce and record the accurate mass: say 11.9726g.
- Remove the sample and container from the balance and tip the contents into a clean, dry beaker (400mL), ensuring that there is no spillage outside the beaker. Do not attempt to wash out the sample tube with water.
- Immediately reweigh the sample tube on the analytical balance: say 2.1564g. This is the mass of the container together with a few crystals of the chemical which have remained in the container. However, you know exacly the mass of the chemical in the beaker: 11.9726 − 2.1564 = 9.8162g of (NH4) 2FeS04.6H20.
- add deionized or distilled water (about 100mL) to the beaker and stir the mixer gently with a clean Pyrex® glass rod untill all the solid has dissolved. Do not splash the solution or you cannot calculate its concentration. Remove the glass rod from the solution, rinsing it with a little distilled water into the solution.
- Clamp a clean volumetric flask for support and place a clean, dry filter funnel in the top supported by a ring, Carefully pour the solution into the volumtric flask, ensuring no spillage of solution by using the technique illustrated in Fig. 4.1 and pouring slowly so that no air-lock is formed and no solution runs down the side of the beaker. When the addition is complete, do not move the beaker from its position over the funnel.
- Rinse the inside of the beaker several times with a distilled water wash bottle to tranfer all of the solution into the volumetric flask, paying particular attention to the 'spout' and glass rod. Then place the beaker aside and lift the funnel from the flask while rinsing it with distilled water. You have now achieved a quantitaive tranfer. Swirl the liquid in the flask to prevent density gradients.
- Make the solution up to the mark using distilled water, stopper
the flask and mix thorouly by gentle inversion (10 times) of the
flask while holding the stopper in place.
You now have a solution (250.00mL0, which contains (NH4) 2FeS04.6H20 (9.8162g).
The concentration of this solution contains 9.8162g of
(NH4)2FeS04.6H20 .
Therefore:
1000.00mL of solution contains (4 × 9.8162) = 39.2648g of (NH4) 2FeS04.6H20.
The concetration of the solution is:
39.2648gL-1 = 39.2648 ÷ 392.14 = 0.1001molL-1 = 0.1001M .
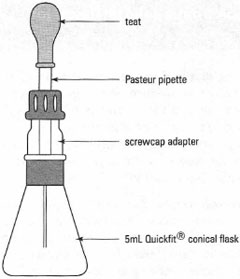
Procedure required with analytical solutions prepared from liquids Many experiments in analytical chemistry, such as chromatography and spectroscopy, require the preparation of a standard solution of a liquid organic compound. Therefore you must know accurately the mass of the liquid. The compound can be dispensed by the methods, provided that the pipette, syringe, etc., is accurate, and thus the mass = volume x density, bearing in mind the temperature factor. Alternatively you can use a weighing bottle as shown in Fig. 4.2. The liquid is placed in the bottle, weighed accurately and then the approximate amount required is added to the volumetric flask containing some solvent. The volumetric flask is stoppered immediately, the weighing bottle reweighed and the weight of liquid dispensed is calculated. The volumetric flask is then made up to the mark and stoppered. You now know the concentration of the standard solution to four decimal places. |
|