Selecting an appropriate buffer
When selecting a buffer, you should be aware of certain limitations to its use. Citric acid and phosphate buffers readily form insoluble complexes with divalent cations, while phosphate can also act as a substrate, activator or inhibitor of certain enzymes. Both of these buffers contain biologically significant quantities of cations, e.g. Na+ or K+. TRIS (Table 7.3) is often toxic to biological systems: owing to its high lipid solubility it can penetrate membranes, uncoupling electron transport reactions in whole cells and isolated organelIes. In addition, it is markedly affected by temperature, with a l0-fold increase in H+ concentration from 4°C to 37°C. A number of zwitterionic molecules (possessing both positive and negative groups) have been introduced to overcome some of the disadvantages of the more traditional buffers. These newer compounds are often referred to as 'Good buffers', to acknowledge the early work of Dr N.B. Good and eo-workers: HEPES is one of the most useful zwitterionic buffers, with a pKa of 7.5 at 25°C.These zwitterionic substances are usually added to water as the free acid: the solution must then be adjusted to the correct pH with a strong alkali, usually NaOH or KOH. Alternatively, they may be used as their sodium or potassium salts, adjusted to the correct pH with a strong acid, e.g. HCI. Consequently, you may need to consider what effects such changes in ion concentration may have in a solution where zwitterions are used as buffers.
Fig. 7.4 shows a number of traditional and zwitterionic buffers and their effective pH ranges. When selecting one of these buffers, aim for a pKa which is in the direction of the expected pH change (Tables 7.2, 7.3). For example, HEPES buffer would be a better choice of buffer than PIPES for use at pH 7.2 for experimental systems where a pH increase is anticipated, while PIPES would be a better choice for where acidification is expected.
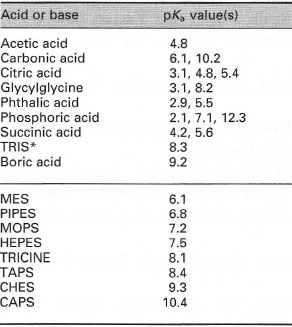 |
| Table 7.3 pKa values at 25°C of some acids and bases (upper section) and some large organic zwitterions (lower section) commonly used in buffer solutions. For polyprotic acids, where more than one proton my dissociate, the pKa values are given for each ionization step. Only the trivial acronyms of the larger molecules are provided: their full names can be obtained from the catalogues of most chemical suppliers. |
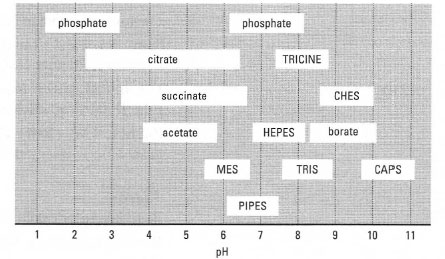 |
| Fig. 7.4 Useful pH ranges of some commonly used buffers. |
Preparation of buffer solutions
Having selected an appropriate buffer, you will need to make up your solution to give the desired pH. You will need to consider two factors:
- The ratio of acid and conjugate base required to give the correct pH.
- The amount of buffering required; buffer capacity depends upon the absolute quantities of acid and base, as well as their relative proportions.
Finally, when preparing a buffer solution based on tabulated information, always confirm the pH with a pH meter before use.
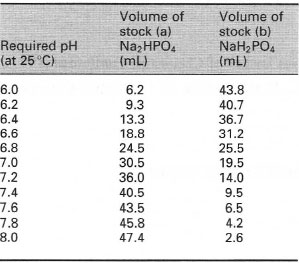 |
| Table 7.4 Preparation of sodium phosphate buffer solutions for use at 25°C. Prepare separate stock solutions of (a) disodium hydrogen phosphate and (b) sodium dihydrogen phosphate, both at 0.2 mol L−1. Buffer solutions (at 0.1 mol L−1) are then prepared at the required pH by mixing together the volume of each stock solution shown in the table, and then diluting to a final volume of 100mL using distilled or deionized water |




