Colligative properties and their use in osmometry
Several properties vary in direct proportion to the effective number of osmotically active solute particles per unit mass of solvent and can be used to determine the osmolality of a solution. These colligative properties include freezing point, boiling point and vapour pressure.An osmometer is an instrument which measures the osmolality of a solution, usually by determining the freezing point depression of the solution in relation to pure water, a technique known as cryoscopic osmometry. A small amount of sample is cooled rapidly and then brought to the freezing point (Fig. 6.1), which is measured by a temperature-sensitive thermistor probe calibrated in mosmol kg-1. An alternative method is used in vapour pressure osmometry, which measures the relative decrease in the vapour pressure produced in the gas phase when a small sample of the solution is equilibrated within a chamber.
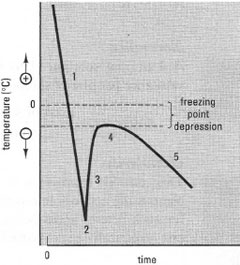 |
| Fig. 6.7 Temperature responses of a cryoscopic osmometer. The response can be subdivided into: 1. initial supercooling, 2. initiation of crystallization, 3. crystallization/freezing 4. plateau, at the freezing point time, 5. slow temperature decrease. |




