Filtration
Filtration is the physical separation of a solid from a liquid and is a process encountered in experimental procedures such as gravimetric analysis, recrystallization, and solvent drying. In principle, the mixture of the solid and liquid is passed through a porous material, filter paper or sintered glass, and the solid is trapped on the porous material while the liquid passes through.The type of filtration equipment you select for use depends upon which of the two components, the solid or the liquid, you are trying to isolate. In general:
- If you wish to isolate the liquid - use gravity filtration.
- If you wish to isolate the solid - use suction (vacuum) filtration.
Gravity filtration
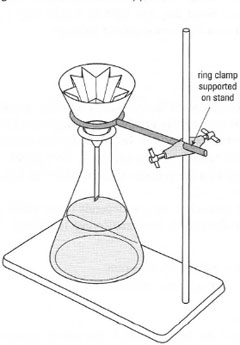
In gravity filtration you need to pass the liquid through the porous material and retain all the unwanted solid in the filter. In general, the best material to use is a filter paper of the appropriate porosity to trap all the solid particles and with the greatest surface area to allow the liquid to pass through quickly. The apparatus required for gravity filtration is shown in Fig. 5.5. The filter funnels are usually made of glass, but if organic solvents are not involved in the filtration, plastic funnels can be used. Glass filter funnels with the pipe cut off are known as 'stemless' filter funnels and have a specific use in hot filtration.
The key to successful gravity filtration is the fluted filter paper. A fluted filter paper decreases the area of contact between the filter paper and the funnel, thus allowing rapid filtration. If you use 'traditional' cone-folded filter paper, note that all sides of the paper are touching the sides of the funnel and on half the filter paper the liquid has to pass through three thicknesses of paper, all of which slow the rate of filtration. Slow filtration can lead to disaster in hot filtration during recrystallization.
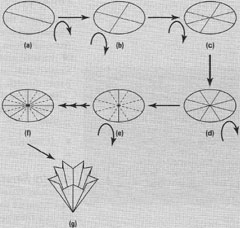
Since filter funnels and filter papers come in different sizes, choose a filter paper of diameter just less than twice the diameter of the funnel. When fluted, the filter paper will be just below the rim of the funnel. There are many ways to fold (flute) a filter paper, but one of the simplest is shown below (steps).
To filter the mixture, swirl the suspension of the solid in the liquid so that there is a fairly even distribution of solid in the liquid, and then pour the mixture into the filter cone, making sure that you do not pour any of the mixture outside the filter paper otherwise you will need to repeat the filtration, and do not overfill the filter cone. Transfer all the mixture in this way and finally wash the last bit of solid and liquid into the filter cone with a small amount of filtered solution and then a little pure solvent.
Steps:
- Fold the filter paper in half (a) open it out then fold into quaters (b), open it and fold into eights (c,d), all in the same directions.
- Turn the paper over and fold each sector in half (e); you are creating sixteenths but with each fold in the opposite direction to (a) (b) (c) and (d)
- Finally, fold the paper into a cone (g) ensuring that all the folds are sharp and that the base of the cone comes to a sharp point.
- The flutes ensure that the filter paper has minimum contact with the filter funnel and the sharp point ensuresthat the liquid flows rapidly out of the cone and out of the funnel
- If ur cone point is blunt it will cover the steam of the funnel and so all the liquid must pass through this part of the filter paper slowing filtration
Suction filtration
This technique is used for the isolation of a solid from a suspension of a solid in a liquid and relies on producing a partial vacuum in the receiving flask. The essential components of a suction filtration system are:
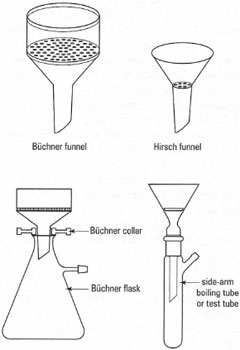
- A ceramic funnel containing a flat perforated plate: there are two types based on size and shape called Buchner funnels or Hirsch funnels. When you are filtering, the perforated plate is covered by a filter paper.
- A receiver flask with a side arm for attachment of the vacuum source. Buchner flasks are conical flasks made from thickened glass, and Hirsch tubes (also known as side-arm boiling tubes or test tubes depending upon size) are capable of withstanding weak vacuum, e.g. a water pump.
- A flexible seal between the ceramic funnel and the receiving vessel: a Buchner collar or filter seal.
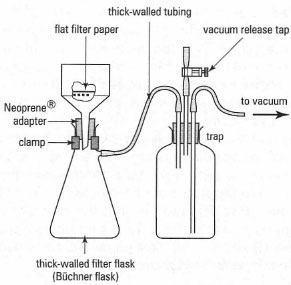
- A source of vacuum, usually a water pump (water aspirator) which is connected to the receiving flask by thick walled rubber tubing (pressure tubing). Sometimes there will be a trap between the water pump and the receiving flask.
The various types of these components are shown in Fig. 5.6: typical apparatus is shown in Fig. 5.7 and the general procedure for suction filtration is described below:
- Select the appropriate size of apparatus based on the amount of solid you expect to isolate and the volume of the liquid to be collected in the receiver flask.
Consider the following points:
A. There is no point in using a large buchner funnel for a small amount os solid since you will collect a layer of solid 'one molecule thick' and be unable to scrape it from the filter paper cleanly. if there is too much solid for the size of the funnel you will have to reapeat the filtration with a second set of apparatus or the solid may not suck dry quickly.
B. If you use the side arm boiling tube to try to collect 100 ml of liquid, you will over fill the tube and liquid,
(i) wii flow into the pressure tubing, contaminating it for your fellow students.
(ii) may fill the intermediate trap, if there is one and you will need to dismental and clean it.
(iii) may be sucked into the water pump causing corrosion and loss of performance.
- Clean and dry all the apparatus to be used:
- Clamp the receiving vessel to support stand:pressure tubing is heavy and even large buchner flacks will fall over do not think that a test tube rack eill hold a side arm boiling tube saftely.
- Place the correct-sized buchner collar in the neck of the receving flask: it should sit well into the neck and fit the funnel to form a good seal.
- Place the funnel into the collar/seal: note that the funnel has a 'point' at the bottom of the stem . Make sure that this 'point' is as far away as possible form the vaccum attachment side arm of the reciever flask, since the filtering liquids runs of this 'point' and if the point is near the vaccume inlet the liquid may be drawn into the side arm and then into the trap or water pump.
- Select a filter paper, whinh fits exactly over the perforation in the base of the funnel. The filter paper should not fold or crease up the sides of the funnel because the solid will be sucked round the edge of the paper into the receiver flask. if the paper does not fit exactly, trim to size with scissors.
- Place the paper into the funnel and wet it with a few drops of liquid - the same liquid which is to be used in the filtration.
- Switch on the tap for the water pump to provide gentle suction. If your system has a trap, don't forget to close the tap on the trap and connect the rubber tubing to the side arm of the reciever. Do nor force the rubber tubing too far onto the side arm - you may need to pull it off quickly if something goes wrong (see 1B and 5). The filter paper will be pulled down onto the preforated plate by the vacuum.
- Turn on the tap to the water pump to the maximum water flow. If you do not do this, the water pump is not working at its maximum efficiency and the vaccum created in yourfiltration system may cause water to be sucked into a trap, or receiving flask, from the water pump. this is called 'suck-back'.
- Swirl the mixture to be filtered and then slowly pour into the Buchner or Hirsch funnle at such a rate so that the flitration is rapid.Note that the rate of filtration may slow as the 'sake ' of solid on the filter becomes thicker.
- To transfer the last of the solid/liquid from its beaker or conical flask into the funnel use a little of the filtrate in the receiving flask. realese the vaccum by opening the tap on the trapor pull off tha vacuum tubing,but do not turn off the tap on the water pump (there is a possibility of 'suck-back' (see 9 above). dis mental the apparatus pour a little of the filtrate into the beaker or conical flask, reassemble the apparatus and continue the filtration. Repeat until all the material has been filtered. Use the filtrate to wash down any of the solid sticking to the sides of the funnel onto the filter 'cake' - it will not dry quickly on the sides of the funnel.
- Release the vacuum, by pulling the vacuum tubing from the flask or opening the tapon the trap and turn down the water pressure on the water pump.Transfer the filtrate to a clean beaker or conical flask. Add a little pure, ice-cold solvent to the filter cake and reconnectthe vacuum to provide gentle suction. this will wash the solid. Turn up the vacuum to maximum and suck air through the solid to dry it as much as possible. If 'cracks' appear in the filter 'cake', close them by pressing gently with a clean spatula and repeat until no more filtrate appears to be sucked out.
- When drying is complete, realese the vacuum, turn off the water tap and remove the filter funnel from the apparatus.The solid is best removes as a complete 'cake' by lifting the edge of the filter paper with spatula, inverting the funnel over a watch-glass of clock-glass and the cake should fall out. peel the filter from the top of the 'cake', break up the 'cake' using a spatula and dry appropriately.
- Evaporate the filtrate to half volume and cool to obtain a second crop of crysta.ls
- Washout and clean all tha apparatus and dispose of the liquid filtrate safely.
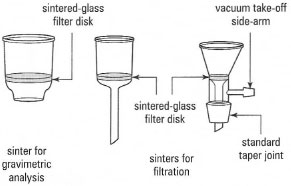
Instead of a filter paper on a porous plate, sintered-glass can be used as the porous material for filtration. Sintered-glass funnels come in various types and sizes (Fig. 5.8) with different porosity (size of holes) of the sintered glass. Sintered glass is also used in crucibles (Fig. 5.8) in gravimetric analysis.
When sintered-glass funnels are used instead of Buchner or Hirsch funnels the solid is collected directly on the sintered glass: no filter paper is used. As a result cleaning sintered-glass funnels is a major problem if the solid has been drawn into the pores of the sintered-glass. Therefore if you are to use a sintered glass funnel, check with your instructor on the appropriate method for cleaning the funnel, before and after use, so that your product will not be contaminated. On the other hand, if particle sizes are large enough to prevent this problem, sintered-glass funnels are very effective in suction filtration.