Cooling
During laboratory work you will be required to carry out experiments at temperatures below room temperature. The most common situations where cooling is required are:- Cooling solutions during recrystallization.
- Completion of precipitation in quantitatative (gravimetric) analysis and preparations.
- Cooling exothermic reactions.
- Carrying out reactions at low temperatures.
The most suitable containers for cooling baths are plastic bowls (ice baths), Pyrex® dishes (solid CO2 baths) and Dewar flasks (solid CO2 and liquid nitrogen baths). If Pyrex® dishes are to be used below -20 QC, an insulated container can be prepared by placing a smaller Pyrex® dish inside a larger one and filling the space between with an insulating material such as cotton wool, cork chips or polyurethane foam chips. Remember that foam chips will dissolve if they come into contact with many organic solvents.
If the temperature of the liquid or solution being cooled is critical, do not assume that the temperature of the liquid or solution is the same as that of the cooling bath: place a thermometer in the flask and remember that for temperatures lower than -5 DC you should use an alcohol thermometer (red thread) or a thermocouple-type thermometer (after checking that the probe will not react with the contents of the flask).
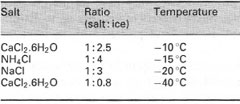
Ice baths A slurry of crushed ice and water can be used to give a cooling bath in the range 0 QC to 5"C. Pure crushed ice does not give good contact with the glassware and inefficient cooling results. If temperatures below 0 QC are required, mixtures of crushed ice and various inorganic salts can be used as shown in Table 5.2. Note that these mixtures contain no liquid and therefore cooling is inefficient and the temperatures indicated in the table are the lowest attainable under ideal conditions. |
|
Solid C02 baths
Solid CO2, when mixed with organic solvents, provides cooling media of temperatures ranging from -15°C to -100°C. Some common mixtures together with the minimum achievable temperatures are shown in Table 5.3. The CO2 and the organic solvent form a 'slush', which gives excellent contact with flasks and, therefore, efficient cooling.
 |
| Table 5.3 Solid CO2 solvent mixtures |
Solid CO2 is supplied as large hard blocks or small quantitres can be prepared from cylinders of liquid CO2 as a 'snow'. Skin contact with solid CO2 will cause frostbite and it must be handled with insulated gloves. The cooling bath must be an insulated container, which will need to be topped up with solid CO2 at regular intervals to maintain the temperature, or, if prolonged cooling is required, a Dewar flask in which the coolant will maintain its temperature for 12 hours or so.
To prepare a solid CO2 cooling bath:
- Choose the appropriate solvent and remember to take into account the hazards associated with its use.
- Break the solid CO2 into small pieces. The CO2 'snow' can be broken with a spatula, but the hard blocks should be wrapped in cloth and then broken into pieces with a wooden or polyethylene mallet.
- Half fill the bath with the solvent and then, using an insulated glove, add small pieces of the solid CO2 until the mixture stops 'boiling' and then add a little more solid CO2 and stir with a glass rod to give a slurry.
- Use an alcohol or thermocouple thermometer to check the temperature of the bath.
- Top up the cooling bath with solid CO2 if the temperature begins to rise.
To prevent the condensation of water into your flask, you should have an inert gas flowing through it and prepare the cold bath around the flask and its contents so that slow cooling occurs. Sudden immersion of a relatively 'hot' flask into the cold bath will cause the bath to 'boil' and air (containing water vapour) will be sucked into the apparatus despite the inert atmosphere. Alternatively a 'loosely packed' CaCl2 guard tube will suffice if cooling is not too rapid.
Cooling probes
These are rigid or flexible metal probes, which are connected to a refrigeration compressor. The probe is placed in the cooling bath and covered with a suitable solvent, which is then cooled to the temperature desired. Cooling probes are commonly found in constant temperature baths, when a temperature below ambient temperature is required, and probes can be used instead of solid CO2 to produce temperatures down to -100 QC. Cooling probes are expensive pieces of equipment; therefore you will find them dedicated to a specific experiment and they are not usually available for basic laboratory operations.