Anaerobic Bacteria
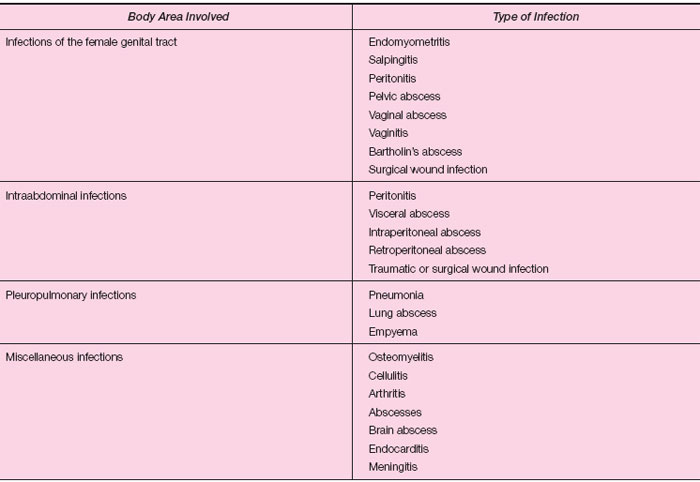
Oligate (strict) anaerobic bacteria cannot grow in the presence of oxygen; therefore, in the laboratory, media containing reducing agents are used to cultivate anaerobes. Agents such as sodium thioglycollate and cystine remove much of the free oxygen present in liquid media. Cooked meat broth is an excellent medium because it contains many reducing agents as well as nutrients. To ensure complete removal of oxygen from the culture environment, cultures are incubated in an “anaerobic jar.”There are two types of anaerobic jars. One type has a lid fitted with an outlet through which air can be evacuated by a vacuum pump and replaced by an oxygen-free gas. A catalyst in the lid catalyzes the reduction and chemical removal of any traces of oxygen that may remain. The other type also requires use of a catalyst in the jar. A foil envelope containing substances that generate hydrogen and CO2 is placed in the jar with the cultures. The envelope is opened, and 10 ml of tap water is pipetted into it. When the jar is closed (the lid is clamped down tightly), the hydrogen given off combines with oxygen, through the mediation of the catalyst, to form water. The CO2 helps to support growth of fastidious anaerobes. A second envelope placed in the jar with the first contains a pad soaked with an oxidation-reduction indicator, for example, methylene blue. When the pad is exposed, the color of the dye indicates whether or not oxygen is present in the jar atmosphere; methylene blue is colorless in the absence of oxygen, blue in its presence. Figure 28.1 illustrates one brand of anaerobic jar (GasPak, B-D Diagnostic Systems) in use. Yet another type of anaerobic system consists of a plastic pouch into which up to 4 petri plates can be placed (fig. 28.2). An anaerobic gas-generating sachet is placed in the bag with the plates. The sachet absorbs oxygen from the pouch and generates CO2 without the need for water. This type of system is convenient when only small numbers of plates are to be incubated anaerobically. In addition, the plates can be viewed for growth through the transparent plastic pouch without exposing the organisms to oxygen. Once sufficient growth is observed, the plates can be opened and the organisms identified.
In recent years, improved techniques for anaerobic culture have been developed in response to an increasing interest in the role of anaerobic bacteria as agents of human infections. Anaerobes have been implicated in a wide variety of infections (see table 28.1) from which multiple species of bacteria are recovered in culture, that is, mixed infections.
The ability of anaerobic organisms to grow in and damage body tissues depends on how well the tissues are oxygenated. Any condition that reduces their oxygen supply, making them anoxic (without oxygen), provides an excellent environment for the growth of anaerobes. Impairment of local circulation because of a crushing wound, hematoma, or other compression leads to tissue anoxia and sets the stage for contaminating anaerobes, if they are present, to cause human infection.
Numerous genera of anaerobic bacteria have been recognized as pathogens, or potential pathogens, and almost all of them are members of the body’s normal flora. The significance of their isolation from a clinical specimen may be difficult to determine, and their pathogenicity is not yet fully understood. Generally, they appear to be “pathogens of opportunity”—that is, given the opportunity to gain access to tissue with impaired blood supply, they may grow and cause enough tissue destruction to establish a local infection. The extent of local or systemic damage may be related to a number of factors, including the properties of microorganism(s) involved, the initial site of infection, and the defense mechanisms of the infected individual. Because anaerobes are part of the normal flora of the body, physicians may have difficulty assessing their importance in a culture taken from an infected area. They must consider whether or not a given isolate may be merely a contaminant from the local normal flora as they evaluate a patient’s clinical condition. The microbiologist can assist by offering directions for the proper collection and transport of specimens, reporting on the predominance of microorganisms present in a culture, and assuring adequate identification of any significant anaerobes that may be isolated.
 |
Figure 28.1 A GasPak jar (BD Bioscience) for cultures to be incubated anaerobically. In this model, the tight-fitting lid contains a catalyst. The large foil envelope has been opened to receive 10 ml of water delivered by a pipette. With the lid clamped in place, hydrogen generated from substances in the large envelope combines with oxygen in the jar’s atmosphere. This combination is mediated by the catalyst and forms water, which condenses on the sides of the jar. Carbon dioxide is also given off by the substances within the large envelope, contributing to the support of growth of fastidious organisms. The smaller envelope has also been opened to expose a pad (arrow) soaked in methylene blue, an indicator used to detect the presence or absence of oxygen. When first exposed, the pad was blue in color. Now it is colorless, indicating that there is no free oxygen left in the jar, for the indicator dye loses its color in the absence of oxygen. The jar now contains an anaerobic atmosphere and can be placed in the incubator. |
Some of the important genera of anaerobic bacteria are listed in table 28.2, most of which are either gram-positive or gram-negative, nonsporing bacilli. With regard to pathogenicity, however, that of certain Clostridium species, which are gram-positive endosporeforming bacilli, has long been recognized and is best understood. Many species in the genus Clostridium are commonly found in the intestinal tract of humans and animals, as well as in the soil, but three are of particular importance in human disease: C. perfringens, C. tetani, and C. botulinum. Each of these is associated with a different and characteristic type of clinical disease.
Clostridium perfringens is an agent of gas gangrene (sometimes in association with other clostridia). If they gain entry into wounded, anaerobic deep tissues, they can multiply rapidly, using tissue carbohydrates, liberating gas, and producing enzymes that cause additional destruction that makes more nutrient available to the bacteria. This can quickly develop into a life-threatening situation if not promptly treated by surgical debridement (removal of dead tissue) and aeration of the injured tissue, and by antimicrobial agents. C. perfringens grows well on blood agar plates in an anaerobic jar, showing characteristic double zones of hemolysis. Its enzymes attack the proteins and carbohydrates of milk, producing “stormy fermentation” of a milk medium, with clotting and gas formation. It ferments a number of carbohydrates with the production of acid and gas. Usually it does not form endospores in ordinary culture media, nor does it do so when growing in tissues.
 |
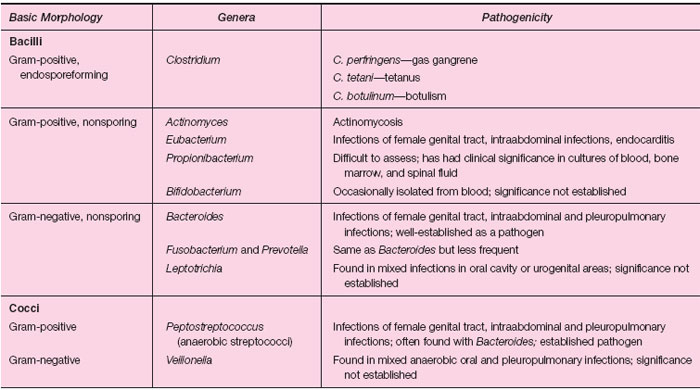
| Figure 28.2 The anaerobe pouch is a convenient system to use when only small numbers of plates are to be incubated anaerobically. The plates are put into the pouch with a gas-generating sachet and an oxygen-reduction indicator. |
 |
| Table 28.1 Infections in which Anaerobic Bacteria Have Been Implicated |
Clostridium botulinum produces an exotoxin that causes the deadly form of food poisoning called botulism. This is not an infectious disease, but a toxic disease. If the endospores of this soil organism survive in processed foods that have been canned or vacuum packed, they may multiply in the anaerobic conditions of the container, elaborating their potent exotoxin in the process of growth. If the food is eaten without further cooking (which would destroy the toxin), the toxin is absorbed and botulism results. The disease is difficult to diagnose bacteriologically, but the incriminated food or the patient’s blood can be tested to demonstrate the toxin’s effect in mice, which confirms the diagnosis.
 |
| Table 28.2 Some Important Genera of Anaerobic Bacteria |
In infant botulism, when endospores of the bacillus (endospores in honey have been implicated in a few cases) are ingested by children under one year of age, the endospores germinate in the child’s intestinal tract in some instances, and the resulting vegetative cells produce toxin. This type of botulism has been implicated in certain cases of sudden infant death syndrome (SIDS).
In this exercise we shall use species of Clostridium to illustrate the general principles of anaerobic culture methods.
| Purpose | To learn basic principles of anaerobic bacteriology |
| Materials | Blood agar plates Thioglycollate broth Tubed skim milk Phenol red broths (glucose, lactose) Blood agar plate cultures of Clostridium perfringens and Clostridium histolyticum Nutrient slant cultures of Pseudomonas aeruginosa and Staphylococcus epidermidis |
Procedures
- Make a Gram stain of each Clostridium culture.
- Select one of the Clostridium cultures and inoculate it on each of two blood agar plates. Label one plate “aerobic,” the other “anaerobic.”
- Inoculate a tube of thioglycollate broth with C. perfringens. Inoculate a second tube of this medium with P. aeruginosa, and a third with S. epidermidis.
- Inoculate a tube of milk with C. perfringens, and a second milk tube with C. histolyticum.
- Inoculate each Clostridium culture into phenol red glucose and lactose, respectively.
- Incubate the blood agar plate labeled “aerobic” in air at 35°C for 24 hours.
- Place all other tubes and plates in the anaerobe jar. (The instructor will demonstrate the method for obtaining an anaerobic atmosphere within the jar.) When it has been set up, the jar is incubated at 35°C for 24 hours. When working with actual clinical specimens, the jar is often not opened until 48 hours, except when Clostridium is highly suspected.
Results
- Indicate the Gram reaction of the Clostridium cultures and illustrate their microscopic morphology.
What stain would you use to determine whether these organisms had produced endospores? ___________
Would you expect to find endospores in the blood agar plate culture of a Clostridium? ___________
Why? ___________ - Examine the thioglycollate broth cultures (do not shake them). On the following figures make a diagram of the
distribution of growth in each tube.
What is your interpretation of the appearance of these tubes? - Examine the blood agar plate cultures, milk tubes, and carbohydrate broths. Record your observations.

State your interpretation of the appearance of the milk cultures.




