Rapid Methods for Bacterial Identification
The biochemical tests performed in the preceding sections are representative of standard methods for bacterial identification. In some instances, it is possible to identify a bacterium correctly by using only a few tests, but more often an extensive biochemical “profile” is needed. Because it is expensive and time consuming to make and keep a wide variety of culture media on hand, many microbiology laboratories now use multimedia identification kits. These are commercially available and are especially useful for identifying the common enteric bacteria. The use of such kits is customarily referred to as an application of “rapid methods,” even though they must be incubated overnight, as usual, before results can be read. Some of them, indeed, are rapid to inoculate, while others permit complete identification within 24 hours.One type of kit, the Enterotube II (BD Diagnostic Systems), is a tube of 12 compartmentalized, conventional agar media that can be inoculated rapidly from a single isolated colony on an agar plate (see colorplate 36). The media provided indicate whether the organism ferments the carbohydrates glucose, lactose, adonitol, arabinose, sorbitol, and dulcitol; produces H2S and/or indole; produces acetylmethylcarbinol; deaminates phenylalanine; splits urea; decarboxylates lysine and/or ornithine; and can use citrate when it is the sole source of carbon in the medium. The mechanism of the other tests provided by the Enterotube II has been described in previous exercises or experiments (17, 18, 24.1).
The API System (bioMérieux Inc.) represents another type of kit for rapid identification of bacteria. This system provides, in a single strip, a series of 20 microtubules (miniature test tubes) of dehydrated media that are rehydrated with a saline suspension of the bacterium to be identified (see colorplate 36). The tests included in the strip determine whether the organism ferments glucose, mannitol, inositol, sorbitol, rhamnose, saccharose, melibiose, and amygdalin; produces indole and H2S; splits urea; breaks down the amino acids tryptophan (same mechanism as phenylalanine), lysine, ornithine, and arginine; produces gelatinase; forms acetylmethylcarbinol from glucose (VP test); and splits the compound o-nitrophenyl-β-D-galactopyranoside (ONPG). The enzyme that acts on ONPG, called β-galactosidase, also is responsible for lactose fermentation. Some bacteria, however, are unable to transport lactose into their cells for breakdown, although they possess β-galactosidase. In lactose broth, therefore, such bacteria fail to display acid production, or do so only after a delay of days or weeks. By contrast, in ONPG medium their β-galactosidase splits the substrate in a matter of hours, producing a bright yellow end product. Thus, ONPG can be used for the rapid demonstration of an organism’s ability to ferment lactose.
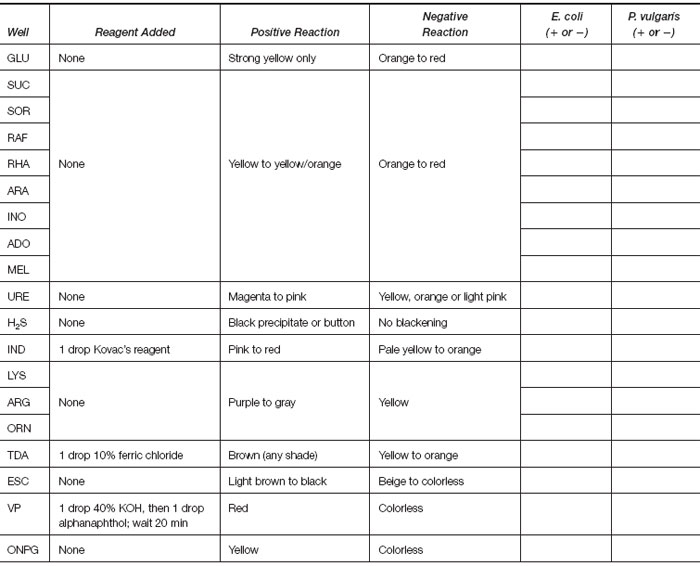
A third type of kit, MicroScan (Dade Behring), consists of a multiwell panel containing dried antimicrobial agents for susceptibility testing and biochemical reagents for identification of enteric and glucose nonfermenting gram-negative bacilli. The wells of the panel are inoculated with a standardized suspension of an organism, incubated for 16 to 24 hours at 35°C, and then read visually or in an automated instrument. In this way, antimicrobial susceptibility testing and organism identification are achieved simultaneously. For enteric organisms, the biochemicals present in the wells test for fermentation of carbohydrates (glucose, sucrose, sorbitol, raffinose, rhamnose, arabinose, inositol, adonitol, melibiose); production of urease, H2S, and indole; breakdown of lysine, arginine, ornithine, tryptophan, and esculin; and VP and ONPG reactions. In addition, tests for glucose nonfermenters include O-F glucose; ability to grow on minimal media containing citrate, malonate, tartrate, and acetamide; and ability to reduce nitrate.
In order to permit more accurate bacterial identification, a computerized recognition system has been devised for each of these three kits that assigns a number to each positive biochemical reaction. These figures are grouped together to give a numerical code to each organism. Unknown bacteria can be identified by looking up the code number provided by their positive reactions in an index book. Different strains of the same bacterium may vary in certain biochemical test results and thus have different code numbers. These variations can sometimes be used as epidemiological markers, in much the same way as phage typing is used to recognize different strains of Staphylococcus aureus.
A further advance is the use of automated instruments to read and interpret the results of both identification and antimicrobial susceptibility tests. The tests are set up in special, clear plastic multiwelled chambers containing a battery of biochemicals and different concentrations of several antimicrobial agents. The plastic chambers are then incubated in the instrument, which periodically scans each biochemical well for changes in the color of pH indicators and scans the antimicrobial agent wells for the presence of turbidity (signifying resistance). At the end of a specific time period, the computer in the instrument interprets all reactions and then the organism identification and its antimicrobial susceptibility results are printed out. Depending on the system used, results can be obtained in as little as 2 to 6 hours.
In this experiment some rapid nonautomated methods for identification of bacteria will be demonstrated.
| Purpose | To observe the biochemical properties of bacteria grown in a multimedia system for rapid identification |
| Materials | Two Enterotubes, API strips, or MicroScan panels (as available) inoculated, respectively, with Escherichia coli and Proteus vulgaris, and incubated for 24 hours. The instructor will demonstrate methods for completing each test in the system. |
Results
- If Enterotubes were inoculated, record the results observed for each organism in the blocks provided under the following
diagram.
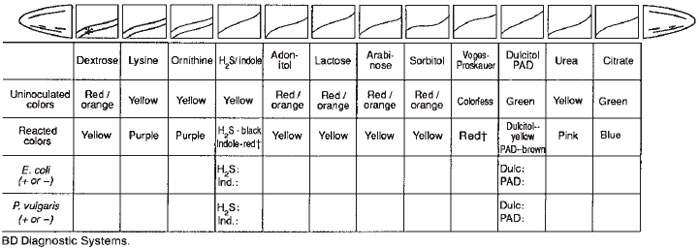

- If API strips were inoculated, record the results observed for each organism in the blocks provided under the following
diagram.
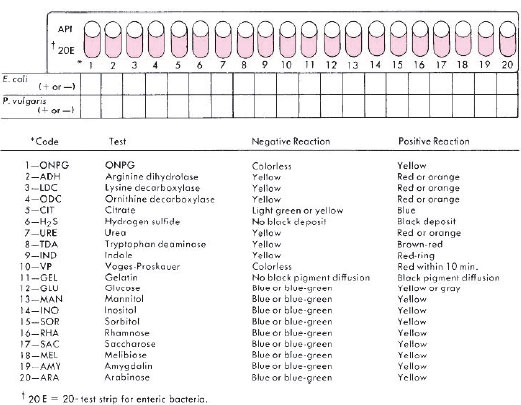
- If the MicroScan panel was used, complete the following table (note that tests included are for enterics only).