Mycoplasmas, rickettsiae, and chlamydiae are classified as true bacteria, but they are extremely
small, and for various reasons cannot be cultured by ordinary bacteriologic methods. The viruses
are the smallest of all microorganisms and are classified separately. The techniques that have developed
over many years for propagating and studying viruses have provided an understanding of their
nature and pathogenicity. The electron microscope, together with elegantly precise biochemical,
physical, molecular, and immunologic procedures, has revealed the structure of viruses and their
role in disease at the cellular level. Prions are proteinaceous infectious particles that cause so-called
slow viral infections because they take many years to develop. Prions are smaller in size than viruses
and are believed to contain no nucleic acids. The means by which such agents can cause disease remains
unknown, but ongoing molecular studies may unravel the answer.
In this exercise we shall review the nature and pathogenicity of these microorganisms.
Procedures
Students will not perform laboratory procedures, but should come to class prepared by assigned reading to discuss the laboratory diagnosis of diseases caused by these agents.
Following is a brief summary of each group.
Mycoplasmas
The mycoplasmas, previously called “pleuropneumonia-like organisms” (PPLO), were first known as etiologic agents of bovine pleuropneumonia. Several species are now recognized, including three that are agents of human infectious disease.
Mycoplasma pneumoniae is the causative organism of “primary atypical pneumonia.” The term implies that the disease is unlike bacterial pneumonias and does not represent a secondary infection by an opportunistic invader, but has a single primary agent. Clinically, mycoplasmal pneumonia resembles an influenza-like illness.
Mycoplasma hominis may be found on healthy mucous membranes, but is also associated with some cases of postpartum fever, pyelonephritis, wound infection, and arthritis.
Ureaplasmas are strains of mycoplasma that produce very tiny colonies and were, for this reason, once called “T-mycoplasmas.”They have been renamed in recognition of their unique possession of the enzyme urease. These mycoplasmas, like M. hominis, are normally found on mucosal surfaces, but have sometimes been associated with urogenital and neonatal infections and female infertility.
Mycoplasmas are extremely pleomorphic (varied in size and shape). They are very thin and plastic because they lack cell walls. For this reason, unlike other bacteria, they can pass through bacterial filters, they do not stain with ordinary dyes, and they are resistant to antimicrobial agents (such as penicillin) that act by interfering with cell wall synthesis.
Laboratory Diagnosis
These organisms can be cultivated on enriched culture media, but on agar media their colonies can be clearly seen only with magnifying lenses. They do not heap on the surface, but extend into and through the agar from the point of inoculation.
Specimens for laboratory diagnosis include sputum, urethral or cervical discharge, synovial fluid, or any material from the site of suspected infection. Cultures require 3 to 10 days of incubation at 35°C. Serological methods are also available for detecting mycoplasmal antibodies in the patient’s serum.
Rickettsiae
The rickettsiae are very small bacteria that survive only when growing and multiplying intracellularly in living cells. In this respect they are like viruses; that is, they are obligate parasites. They have a cell wall similar to that of other bacteria, which can be stained with special stains so that their morphology can be studied with the light microscope.
Certain arthropods, such as ticks, mites, or lice, are the natural reservoirs of rickettsiae. They are transmitted to humans by the bite of such insects, by rubbing infected insect feces into skin (for example, after a bite), or by inhaling aerosols contaminated by infected insects. The most important rickettsial pathogens are Rickettsia prowazekii (epidemic typhus), Rickettsia rickettsii (Rocky Mountain spotted fever), Rickettsia akari (rickettsialpox), and Coxiella burnetii (Q fever).
Ehrlichia species are classified in the same family as rickettsiae. They are recently recognized agents of several diseases, especially in Japan and the United States. Some ehrlichiae are transmitted by ticks. Ehrlichiae sennetsu, common in Japan, produces a disease resembling infectious mononucleosis. Ehrlichia chaffeensis, a tick-borne disease in the United States, produces symptoms similar to Rocky Mountain spotted fever, but without the rash.
Following is a list of the major groups of the rickettsial family and the diseases they cause.
Laboratory Diagnosis
In the laboratory, rickettsiae can be propagated only in cell culture or in intact animals, such as chick embryos, mice, and guinea pigs. They are identified by their growth characteristics, by the type of injury they create in cells or animals, and by serological means. Serological diagnosis of rickettsial diseases can also be made by identifying patients’ serum antibodies.
Chlamydiae
The chlamydiae are intermediate in size between rickettsiae and the largest viruses, which they were once thought to be. They are now recognized as true bacteria because of the structure and composition of their cell walls (the term chlamydia means “thick-walled”) and because their basic reproductive mechanism is of the bacterial type. They are nonmotile, coccoid organisms that, like the rickettsiae, are obligate parasites. Their intracellular life is characterized by a unique developmental cycle. When first taken up by a parasitized cell, the chlamydial organism becomes enveloped within a membranous vacuole. This “elementary body” then reorganizes and enlarges, becoming what is called a “reticulate body.”The latter, still within its vacuole, then begins to divide repeatedly by binary fission, producing a mass of small particles termed an “inclusion body” (see colorplate 40). Eventually the particles are freed from the cell, and each of the new small particles (again called elementary bodies) may then infect another cell, beginning the cycle again.
Three chlamydial species are responsible for human disease. Chlamydia psittaci causes ornithosis, or psittacosis (“parrot” fever), a pneumonia transmitted to humans usually by certain pet birds. Chlamydia trachomatis currently is the most common bacterial agent of sexually transmitted disease; the infection often is referred to as nongonococcal urethritis. In addition, this species causes a less common sexually transmitted disease, lymphogranuloma venereum; infant pneumonitis; and trachoma, a severe eye disease that can lead to blindness. Chlamydia pneumoniae produces a variety of respiratory diseases, especially in young adults. Because of difficulties growing it, the organism was identified only during the 1980s. Undoubtedly it has been causing disease for many years, if not for centuries.
Laboratory Diagnosis
Chlamydia psittaci and Chlamydia pneumoniae are almost always diagnosed by serological means. Cell culture methods are available for growing Chlamydia psittaci, but isolating this organism in culture is hazardous and performed only in laboratories with specialized containment facilities.
Cell culture methods are also available for isolating Chlamydia trachomatis, but they are cumbersome, performed only in specialized laboratories, and generally reserved for cases of suspected child abuse. The development of nucleic acid probe and amplification assays has greatly aided diagnosis of this common sexually transmitted disease pathogen. In addition to genital specimens, eye, urine, and infant respiratory specimens may be tested, depending on the system used.
Viruses
Viruses are infectious agents that reproduce only within intact living cells. They are so small and simple in structure, and so limited in almost all activity, that they challenge our definitions of life and of living organisms. The smallest are comparable in size to a large molecule. Structurally, they are not true cells but subunits, containing only an essential nucleic acid wrapped in a protein coat, or capsid. The electron microscope reveals that they have various shapes, some being merely globular, others rodlike, and some with a head and tailpiece resembling a tadpole. When viruses are purified, their crystalline forms may have distinctive patterns. An intact, noncrystallized virus particle is called a virion.
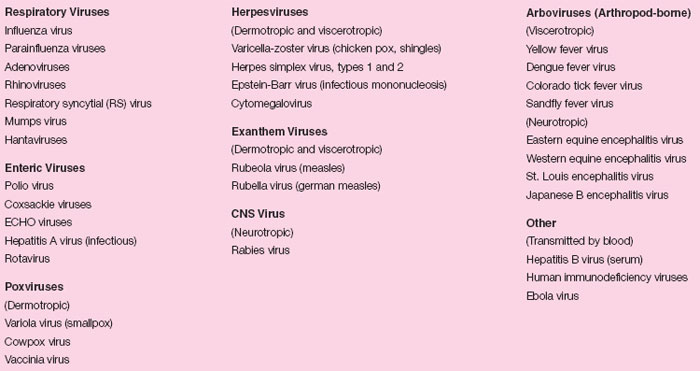
There are many ways to classify viruses: on the basis of their chemical composition, morphology, and similar measurable properties. From the clinical point of view, it seems practical to classify them on the basis of the type of disease they produce. This, in turn, is based on their differing affinities for particular types of host cells or tissues. Thus, we speak of neurotropic viruses as those that have a specific affinity for cells of the nervous system. Dermotropic viruses affect the epithelial cells of the skin, and viscerotropic viruses parasitize internal organs, notably the liver. Enteric viruses are so-called because they enter the body through the gastrointestinal tract. Their primary disease effects are exerted elsewhere, however, when they disseminate from this site of initial entry. The term arbovirus is used for those viruses that exist in arthropod reservoirs and are transmitted to humans by their biting insect hosts (i.e., they are arthropodborne). Still other viruses, such as the human immunodeficiency virus, have effects on multiple body systems. In table 30.1, some important viruses are grouped in a clinical and epidemiological classification that reflects either their route of transmission or the type of disease they cause in humans.
Laboratory Diagnosis
A variety of methods may be used for the laboratory diagnosis of viral infections. These include isolation of the virus in cell culture; direct examination of clinical material to detect viral particles, antigens, or nucleic acids; cytohistological (cellular) evidence of infection; and serological assays to assess an individual’s antibody response to infection. No single laboratory approach is completely reliable in diagnosing all viral infections. Therefore, the use of any one or a combination of these methods may be needed to establish a specific viral etiology of disease. The choice of method may be determined by several factors, including knowledge of the pathogenesis of the suspected viral agent, the stage of the illness, and the availability of various laboratory methods for the particular viral infection suspected.
Cell Culture
Viruses are obligate, intracellular parasites that require metabolically active cells for their replication. Most can be cultivated in mammalian cell cultures, embryonated chicken eggs, or laboratory animals, such as mice. In many clinical laboratories, cell culture has supplanted the other systems for isolating most viruses. Unfortunately, a single, universal cell culture suitable for the recovery of all viruses is not available. Because of this, several different cell culture lines are used to optimize recovery of the viral agents most common in human disease. These include Rhesus monkey kidney cells, rabbit kidney cells, human embryonic lung cells (called WI-38 cells), and human epidermoid carcinoma cells of the larynx or lung, called HEp-2 or A549 cells, respectively. These cell lines are cultivated in glass or plastic tubes or flasks using specially formulated cell culture media. The cells adhere to the glass surface and produce a confluent, single layer of growth known as a cell monolayer (see fig. 30.1).
The ability of a virus to infect a particular cell line depends on the presence of specific receptor sites on the cell membrane to which the virus can attach. Attachment is followed by virus entry into the cell. The presence or absence of certain receptor sites on the cell membrane surface determines the susceptibility or sensitivity of that particular cell line to viral infection.
Once a virus infects a mammalian cell, it may induce certain morphologic changes in the typical appearance of the cells, known as a cytopathic effect or CPE (see fig. 30.1). Some types of CPE caused by different viruses include generalized cell rounding, syncytia formation (fusion of cells), and plaque formation (lysis of cells). Importantly, the type of cell line infected and resultant CPE produced are extremely useful in providing the identity of the particular virus isolated. The CPE may take from 1 to 25 days to develop, depending on the virus isolated.
Certain groups of viruses, such as the influenza and parainfluenza viruses, may not produce CPE when they infect cell cultures, and thus, cell monolayers infected with them appear normal morphologically. A unique property of these viruses, however, is their ability to produce hemagglutinins, which are proteins projecting from the envelopes of the viruses and present in the membranes of infected cells. Hemagglutinins have the ability to adhere to erythrocytes in a process known as hemadsorption, which is used to screen certain cell cultures for the presence of influenza and parainfluenza viruses. This test is performed by overlaying the cell monolayer with a suspension of guinea pig erythrocytes, then examining for the presence of hemadsorption after 30 minutes. Adherence of the guinea pig erythrocytes to the cell monolayer is regarded as a positive test. Influenza and parainfluenza viruses are the most commonly isolated hemadsorbing viruses, but mumps virus also gives a positive reaction.
Despite the availability of a large number of different cell culture lines, a number of clinically important viruses cannot be grown using these conventional methods. The Epstein-Barr virus (the cause of infectious mononucleosis) and human immunodeficiency virus (the cause of AIDS) require human white blood cells for growth. Other viruses, such as some coxsackie A viruses, rabies virus, and arboviruses are best isolated in mice. Because of the highly specialized nature of these procedures, such methods are generally performed only in reference laboratories. In addition, some viruses (e.g., hepatitis viruses and rotavirus) cannot be cultivated at all. Alternative procedures such as electron microscopy, antigen detection assays, or serology are used for the diagnosis of these viral infections.
Direct Specimen Assays
Immunologic assays, such as immunofluorescence and enzyme immunoassay, are used to detect viral antigens, and nucleic acid amplification techniques are used to detect viral nucleic acids directly in patient specimens. Antigen detection assays are available for a number of different viruses including respiratory syncytial virus, herpes simplex virus, influenza A and B viruses, rotavirus, and adenovirus. Currently, nucleic acid amplification assays are limited to the detection of human papillomavirus although assays for quantifying blood levels of viruses such as HIV are available. If viral products are detected, the laboratory diagnosis of infection is established and the need to perform viral culture is eliminated. Results are often available within 10 to 60 minutes.
Cytohistological Examination
The earliest nonculture laboratory method used for viral diagnosis was screening for characteristic changes in infected human cells and tissues. Examination of cell smears or tissue sections stained with special tissue stains may reveal characteristic viral inclusion bodies that represent “footprints” of viral replication and are suggestive of certain viral infections. However, the diagnostic value of such an approach is limited because sensitivity is low (50 to 70%) compared with other available methods. The major application of this method is for the diagnosis of infections caused by viruses such as molluscum contagiosum (the cause of genital warts), which are not culturable. However, a gene amplification method is now commercially available for detecting these viruses in clinical samples.
Electron Microscopy
Electron microscopy is a powerful tool for the study of viral morphology and size but is of limited availability in most diagnostic laboratories. Direct electron microscopy also requires specimens containing high titers (≥107 per ml) of viral particles. The major diagnostic application of electron microscopy is for the detection of certain nonculturable viruses, particularly those that cause gastroenteritis (e.g., caliciviruses, astroviruses, and rotavirus).
Serology
Serological tests to identify patient’s antibodies are described in more detail in Serological Identification of Patients’ Antibodies. A variety of serological tests, however, are available for the diagnosis of many viral infections.
These involve the examination of two serum specimens (acute and convalescent sera spaced at least 2–4 weeks apart) to detect a significant change in antibody titer. Serology is extremely useful for the diagnosis of infections caused by the various hepatitis viruses.
Prions
Prion is a shorthand term for proteinaceous infectious particles. They are smaller in size than viruses and are believed to contain neither DNA nor RNA. Prions cause slow neurodegenerative diseases known as spongiform encephalopathies. They are classified as slow viral infections because 20 to 30 years following exposure to the agent may elapse before symptoms of infection develop in the patient. Creutzfeldt-Jakob disease and kuru are examples of human prion disease.
In recent years, prions have attracted international scientific and public attention due to the outbreak of bovine spongiform encephalopathy, also known as “mad cow disease,” in Great Britain and some other European countries. Mad cow disease causes infection primarily in cattle and sheep, but human infections can result from eating infected animal meat. Prions are highly resistant to destruction and are not inactivated by thorough cooking of infected animal products. No treatments are available for diseases caused by prions and the diseases are universally fatal, with death usually occurring within one year of the onset of symptomatic disease.
The diagnosis of prion infection is problematic. Currently, there is no clinical laboratory method available to establish the diagnosis. Instead, diagnosis is based on clinical suspicion confirmed by demonstrating characteristic spongiform changes (spongelike holes) in histological sections of brain tissue, usually postmortem. Recent evidence indicates that these spongiform changes may be seen also in more readily accessible tonsillar tissue.
In this exercise we shall review the nature and pathogenicity of these microorganisms.
| Purpose | To learn the role of mycoplasmas, rickettsiae, chlamydiae, viruses, and prions in disease and to review some laboratory procedures for recognizing them |
| Materials | Audiovisual or reading materials illustrating each group Diagram of the electron microscope |
Procedures
Students will not perform laboratory procedures, but should come to class prepared by assigned reading to discuss the laboratory diagnosis of diseases caused by these agents.
Following is a brief summary of each group.
Mycoplasmas
The mycoplasmas, previously called “pleuropneumonia-like organisms” (PPLO), were first known as etiologic agents of bovine pleuropneumonia. Several species are now recognized, including three that are agents of human infectious disease.
Mycoplasma pneumoniae is the causative organism of “primary atypical pneumonia.” The term implies that the disease is unlike bacterial pneumonias and does not represent a secondary infection by an opportunistic invader, but has a single primary agent. Clinically, mycoplasmal pneumonia resembles an influenza-like illness.
Mycoplasma hominis may be found on healthy mucous membranes, but is also associated with some cases of postpartum fever, pyelonephritis, wound infection, and arthritis.
Ureaplasmas are strains of mycoplasma that produce very tiny colonies and were, for this reason, once called “T-mycoplasmas.”They have been renamed in recognition of their unique possession of the enzyme urease. These mycoplasmas, like M. hominis, are normally found on mucosal surfaces, but have sometimes been associated with urogenital and neonatal infections and female infertility.
Mycoplasmas are extremely pleomorphic (varied in size and shape). They are very thin and plastic because they lack cell walls. For this reason, unlike other bacteria, they can pass through bacterial filters, they do not stain with ordinary dyes, and they are resistant to antimicrobial agents (such as penicillin) that act by interfering with cell wall synthesis.
Laboratory Diagnosis
These organisms can be cultivated on enriched culture media, but on agar media their colonies can be clearly seen only with magnifying lenses. They do not heap on the surface, but extend into and through the agar from the point of inoculation.
Specimens for laboratory diagnosis include sputum, urethral or cervical discharge, synovial fluid, or any material from the site of suspected infection. Cultures require 3 to 10 days of incubation at 35°C. Serological methods are also available for detecting mycoplasmal antibodies in the patient’s serum.
Rickettsiae
The rickettsiae are very small bacteria that survive only when growing and multiplying intracellularly in living cells. In this respect they are like viruses; that is, they are obligate parasites. They have a cell wall similar to that of other bacteria, which can be stained with special stains so that their morphology can be studied with the light microscope.
Certain arthropods, such as ticks, mites, or lice, are the natural reservoirs of rickettsiae. They are transmitted to humans by the bite of such insects, by rubbing infected insect feces into skin (for example, after a bite), or by inhaling aerosols contaminated by infected insects. The most important rickettsial pathogens are Rickettsia prowazekii (epidemic typhus), Rickettsia rickettsii (Rocky Mountain spotted fever), Rickettsia akari (rickettsialpox), and Coxiella burnetii (Q fever).
Ehrlichia species are classified in the same family as rickettsiae. They are recently recognized agents of several diseases, especially in Japan and the United States. Some ehrlichiae are transmitted by ticks. Ehrlichiae sennetsu, common in Japan, produces a disease resembling infectious mononucleosis. Ehrlichia chaffeensis, a tick-borne disease in the United States, produces symptoms similar to Rocky Mountain spotted fever, but without the rash.
Following is a list of the major groups of the rickettsial family and the diseases they cause.
- Typhus group
- Epidemic typhus
- Murine typhus
- Scrub typhus (tsutsugamushi fever)
- Spotted fever group
- Rocky Mountain spotted fever
- Rickettsialpox
- Boutonneuse fever
- Coxiella (The genus Coxiella is undergoing reclassification and may be removed from the rickettsial family.)
- Q fever
- Ehrlichiae
- Ehrlichiosis
- Sennetsu fever (Japan)
Laboratory Diagnosis
In the laboratory, rickettsiae can be propagated only in cell culture or in intact animals, such as chick embryos, mice, and guinea pigs. They are identified by their growth characteristics, by the type of injury they create in cells or animals, and by serological means. Serological diagnosis of rickettsial diseases can also be made by identifying patients’ serum antibodies.
Chlamydiae
The chlamydiae are intermediate in size between rickettsiae and the largest viruses, which they were once thought to be. They are now recognized as true bacteria because of the structure and composition of their cell walls (the term chlamydia means “thick-walled”) and because their basic reproductive mechanism is of the bacterial type. They are nonmotile, coccoid organisms that, like the rickettsiae, are obligate parasites. Their intracellular life is characterized by a unique developmental cycle. When first taken up by a parasitized cell, the chlamydial organism becomes enveloped within a membranous vacuole. This “elementary body” then reorganizes and enlarges, becoming what is called a “reticulate body.”The latter, still within its vacuole, then begins to divide repeatedly by binary fission, producing a mass of small particles termed an “inclusion body” (see colorplate 40). Eventually the particles are freed from the cell, and each of the new small particles (again called elementary bodies) may then infect another cell, beginning the cycle again.
Three chlamydial species are responsible for human disease. Chlamydia psittaci causes ornithosis, or psittacosis (“parrot” fever), a pneumonia transmitted to humans usually by certain pet birds. Chlamydia trachomatis currently is the most common bacterial agent of sexually transmitted disease; the infection often is referred to as nongonococcal urethritis. In addition, this species causes a less common sexually transmitted disease, lymphogranuloma venereum; infant pneumonitis; and trachoma, a severe eye disease that can lead to blindness. Chlamydia pneumoniae produces a variety of respiratory diseases, especially in young adults. Because of difficulties growing it, the organism was identified only during the 1980s. Undoubtedly it has been causing disease for many years, if not for centuries.
Laboratory Diagnosis
Chlamydia psittaci and Chlamydia pneumoniae are almost always diagnosed by serological means. Cell culture methods are available for growing Chlamydia psittaci, but isolating this organism in culture is hazardous and performed only in laboratories with specialized containment facilities.
Cell culture methods are also available for isolating Chlamydia trachomatis, but they are cumbersome, performed only in specialized laboratories, and generally reserved for cases of suspected child abuse. The development of nucleic acid probe and amplification assays has greatly aided diagnosis of this common sexually transmitted disease pathogen. In addition to genital specimens, eye, urine, and infant respiratory specimens may be tested, depending on the system used.
Viruses
Viruses are infectious agents that reproduce only within intact living cells. They are so small and simple in structure, and so limited in almost all activity, that they challenge our definitions of life and of living organisms. The smallest are comparable in size to a large molecule. Structurally, they are not true cells but subunits, containing only an essential nucleic acid wrapped in a protein coat, or capsid. The electron microscope reveals that they have various shapes, some being merely globular, others rodlike, and some with a head and tailpiece resembling a tadpole. When viruses are purified, their crystalline forms may have distinctive patterns. An intact, noncrystallized virus particle is called a virion.
There are many ways to classify viruses: on the basis of their chemical composition, morphology, and similar measurable properties. From the clinical point of view, it seems practical to classify them on the basis of the type of disease they produce. This, in turn, is based on their differing affinities for particular types of host cells or tissues. Thus, we speak of neurotropic viruses as those that have a specific affinity for cells of the nervous system. Dermotropic viruses affect the epithelial cells of the skin, and viscerotropic viruses parasitize internal organs, notably the liver. Enteric viruses are so-called because they enter the body through the gastrointestinal tract. Their primary disease effects are exerted elsewhere, however, when they disseminate from this site of initial entry. The term arbovirus is used for those viruses that exist in arthropod reservoirs and are transmitted to humans by their biting insect hosts (i.e., they are arthropodborne). Still other viruses, such as the human immunodeficiency virus, have effects on multiple body systems. In table 30.1, some important viruses are grouped in a clinical and epidemiological classification that reflects either their route of transmission or the type of disease they cause in humans.
 |
| Table 30.1 Clinical and Epidemiological Classification of Some Clinically Important Viruses |
Laboratory Diagnosis
A variety of methods may be used for the laboratory diagnosis of viral infections. These include isolation of the virus in cell culture; direct examination of clinical material to detect viral particles, antigens, or nucleic acids; cytohistological (cellular) evidence of infection; and serological assays to assess an individual’s antibody response to infection. No single laboratory approach is completely reliable in diagnosing all viral infections. Therefore, the use of any one or a combination of these methods may be needed to establish a specific viral etiology of disease. The choice of method may be determined by several factors, including knowledge of the pathogenesis of the suspected viral agent, the stage of the illness, and the availability of various laboratory methods for the particular viral infection suspected.
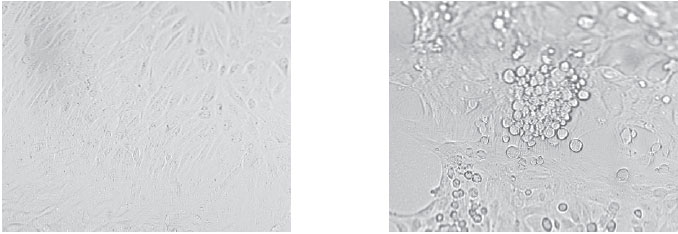 |
| Figure 30.1 Cell culture of adenovirus. The uninoculated cells on the left form an even monolayer (one cell thick) in the culture tube. Once the cells are infected with virus (right), they undergo a characteristic cytopathic effect, becoming enlarged, granular in appearance, and aggregated into irregular clusters. |
Cell Culture
Viruses are obligate, intracellular parasites that require metabolically active cells for their replication. Most can be cultivated in mammalian cell cultures, embryonated chicken eggs, or laboratory animals, such as mice. In many clinical laboratories, cell culture has supplanted the other systems for isolating most viruses. Unfortunately, a single, universal cell culture suitable for the recovery of all viruses is not available. Because of this, several different cell culture lines are used to optimize recovery of the viral agents most common in human disease. These include Rhesus monkey kidney cells, rabbit kidney cells, human embryonic lung cells (called WI-38 cells), and human epidermoid carcinoma cells of the larynx or lung, called HEp-2 or A549 cells, respectively. These cell lines are cultivated in glass or plastic tubes or flasks using specially formulated cell culture media. The cells adhere to the glass surface and produce a confluent, single layer of growth known as a cell monolayer (see fig. 30.1).
The ability of a virus to infect a particular cell line depends on the presence of specific receptor sites on the cell membrane to which the virus can attach. Attachment is followed by virus entry into the cell. The presence or absence of certain receptor sites on the cell membrane surface determines the susceptibility or sensitivity of that particular cell line to viral infection.
Once a virus infects a mammalian cell, it may induce certain morphologic changes in the typical appearance of the cells, known as a cytopathic effect or CPE (see fig. 30.1). Some types of CPE caused by different viruses include generalized cell rounding, syncytia formation (fusion of cells), and plaque formation (lysis of cells). Importantly, the type of cell line infected and resultant CPE produced are extremely useful in providing the identity of the particular virus isolated. The CPE may take from 1 to 25 days to develop, depending on the virus isolated.
Certain groups of viruses, such as the influenza and parainfluenza viruses, may not produce CPE when they infect cell cultures, and thus, cell monolayers infected with them appear normal morphologically. A unique property of these viruses, however, is their ability to produce hemagglutinins, which are proteins projecting from the envelopes of the viruses and present in the membranes of infected cells. Hemagglutinins have the ability to adhere to erythrocytes in a process known as hemadsorption, which is used to screen certain cell cultures for the presence of influenza and parainfluenza viruses. This test is performed by overlaying the cell monolayer with a suspension of guinea pig erythrocytes, then examining for the presence of hemadsorption after 30 minutes. Adherence of the guinea pig erythrocytes to the cell monolayer is regarded as a positive test. Influenza and parainfluenza viruses are the most commonly isolated hemadsorbing viruses, but mumps virus also gives a positive reaction.
Despite the availability of a large number of different cell culture lines, a number of clinically important viruses cannot be grown using these conventional methods. The Epstein-Barr virus (the cause of infectious mononucleosis) and human immunodeficiency virus (the cause of AIDS) require human white blood cells for growth. Other viruses, such as some coxsackie A viruses, rabies virus, and arboviruses are best isolated in mice. Because of the highly specialized nature of these procedures, such methods are generally performed only in reference laboratories. In addition, some viruses (e.g., hepatitis viruses and rotavirus) cannot be cultivated at all. Alternative procedures such as electron microscopy, antigen detection assays, or serology are used for the diagnosis of these viral infections.
Direct Specimen Assays
Immunologic assays, such as immunofluorescence and enzyme immunoassay, are used to detect viral antigens, and nucleic acid amplification techniques are used to detect viral nucleic acids directly in patient specimens. Antigen detection assays are available for a number of different viruses including respiratory syncytial virus, herpes simplex virus, influenza A and B viruses, rotavirus, and adenovirus. Currently, nucleic acid amplification assays are limited to the detection of human papillomavirus although assays for quantifying blood levels of viruses such as HIV are available. If viral products are detected, the laboratory diagnosis of infection is established and the need to perform viral culture is eliminated. Results are often available within 10 to 60 minutes.
Cytohistological Examination
The earliest nonculture laboratory method used for viral diagnosis was screening for characteristic changes in infected human cells and tissues. Examination of cell smears or tissue sections stained with special tissue stains may reveal characteristic viral inclusion bodies that represent “footprints” of viral replication and are suggestive of certain viral infections. However, the diagnostic value of such an approach is limited because sensitivity is low (50 to 70%) compared with other available methods. The major application of this method is for the diagnosis of infections caused by viruses such as molluscum contagiosum (the cause of genital warts), which are not culturable. However, a gene amplification method is now commercially available for detecting these viruses in clinical samples.
Electron Microscopy
Electron microscopy is a powerful tool for the study of viral morphology and size but is of limited availability in most diagnostic laboratories. Direct electron microscopy also requires specimens containing high titers (≥107 per ml) of viral particles. The major diagnostic application of electron microscopy is for the detection of certain nonculturable viruses, particularly those that cause gastroenteritis (e.g., caliciviruses, astroviruses, and rotavirus).
Serology
Serological tests to identify patient’s antibodies are described in more detail in Serological Identification of Patients’ Antibodies. A variety of serological tests, however, are available for the diagnosis of many viral infections.
These involve the examination of two serum specimens (acute and convalescent sera spaced at least 2–4 weeks apart) to detect a significant change in antibody titer. Serology is extremely useful for the diagnosis of infections caused by the various hepatitis viruses.
Prions
Prion is a shorthand term for proteinaceous infectious particles. They are smaller in size than viruses and are believed to contain neither DNA nor RNA. Prions cause slow neurodegenerative diseases known as spongiform encephalopathies. They are classified as slow viral infections because 20 to 30 years following exposure to the agent may elapse before symptoms of infection develop in the patient. Creutzfeldt-Jakob disease and kuru are examples of human prion disease.
In recent years, prions have attracted international scientific and public attention due to the outbreak of bovine spongiform encephalopathy, also known as “mad cow disease,” in Great Britain and some other European countries. Mad cow disease causes infection primarily in cattle and sheep, but human infections can result from eating infected animal meat. Prions are highly resistant to destruction and are not inactivated by thorough cooking of infected animal products. No treatments are available for diseases caused by prions and the diseases are universally fatal, with death usually occurring within one year of the onset of symptomatic disease.
The diagnosis of prion infection is problematic. Currently, there is no clinical laboratory method available to establish the diagnosis. Instead, diagnosis is based on clinical suspicion confirmed by demonstrating characteristic spongiform changes (spongelike holes) in histological sections of brain tissue, usually postmortem. Recent evidence indicates that these spongiform changes may be seen also in more readily accessible tonsillar tissue.




