Atomic spectroscopy
Atoms of certain metals will absorb and emit radiation of specific wavelengths when heated in a flame, in direct proportion to the number of atoms present. Atomic spectrophotometric techniques measure the absorption or emission of particular wavelengths of UV and visible light, to identify and quantify such metals.Flame atomic emission spectrophotometry (or flame photometry)
The principal components of a flame photometer are shown in Fig. 26.5. A liquid sample is converted into an aerosol in a nebulizer (atomizer) before being introduced into the flame, where a small proportion (typically less than I in 10000) of the atoms will be raised to a higher energy level, releasing this energy as light of a specific wavelength, which is passed through a filter to a photocell detector. Flame photometry can be used to measure the alkali metal ions K+, Na+ and Ca2+ in, for example, biological fluids and water samples (Box 26.2).
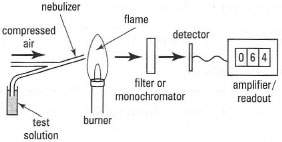 |
| Fig. 26.5 Components of a flame photometer. |
Atomic absorption spectroscopy
This technique is applicable to a broad range of metal ions, including those of Pb, Cu, Zn, etc. It relies on the absorption of light of a specific wavelength by atoms dispersed in a flame. The appropriate wavelength is provided by a hollow cathode lamp, coated with the element to be analysed, focused through the flame and onto the detector. When the sample is introduced into the flame, it will decrease the light detected in direct proportion to the amount of metal present. Practical advantages over flame photometry include:
- improved sensitivity;
- increased precision;
- decreased interference.




