Basic spectroscopy
Light is most strictly defined as that part of the spectrum of electromagnetic
radiation detected by the human eye. However, the term is also applied to
radiation just outside that visible range, e.g. ultraviolet (UV) and infrared
(IR) 'light'. Electromagnetic radiation is emitted by the sun and by other
sources (e.g. an incandescent lamp) and the electromagnetic spectrum is a
broad band of radiation, ranging from cosmic rays to radio waves (Fig. 26.1).
Most chemical experiments involve measurements within the UV, visible and
IR regions (generally, within the wavelength range 200-1000 nm).
Radiation has the characteristics of a particle and of a vibrating wave,
travelling in discrete particulate units, or 'packets', termed photons. A
quantum is the amount of energy contained within a single photon (it is
important not to confuse these two terms, although they are sometimes used
interchangeably in the literature). In some circumstances, it is appropriate to
measure light in terms of the number of photons, usually expressed directly in
moles (6.02 × 10
23 photons = 1 mol). Alternatively, the energy content
(power) may be measured (e.g. in W m
−2). Radiation also behaves as a
vibrating electrical and magnetic field moving in a particular direction, with
the magnetic and electrical components vibrating perpendicular to one
another and perpendicular to the direction of travel. The wave nature of
radiation gives rise to the concepts of wavelength (
λ, usually measured in
nm), frequency (
ν, measured in s
−1, but often recorded in hertz, Hz), speed
(
c, the speed of electromagnetic radiation, which is 3 × 10
8 m s
−1 in a
vacuum), and direction. In other words, radiation is a vector quantity, where:
Sometimes, it is necessary to rearrange the equation such that:
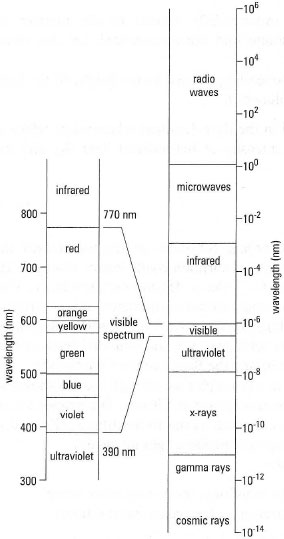 |
| Fig. 26.1 The electromagnetic spectrum. |





