Capillary electrophoresis
The technique of capillary electrophoresis (CE) combines the high resolving power of electrophoresis with the speed and versatility of HPLC. The technique largely overcomes the major problem of carrying out electrophoresis without a supporting medium, i.e. poor resolution due to convection currents and diffusion. A capillary tube has a high surface-area-to-volume ratio, and consequently the heat generated as a result of the applied electric current is rapidly dissipated. A further advantage is that very small sample volumes (5 - 10 nL) can be analysed. The versatility of CE is demonstrated by its use in the separation of a range of molecules, e.g. amino acids, proteins, nucleic acids, drugs, vitamins, organic acids and inorganic ions; CE can even separate neutral species, e.g. steroids, aromatic hydrocarbons.The components of a typical CE apparatus are shown in Fig. 33.3. The capillary is made of fused silica and externally coated with a polymer for mechanical strength. The internal diameter is usually 25-50,µm, a compromise between efficient heat dissipation and the need for a light path that is not too short for detection using UV/visible spectrophotometry. A gap in the polymer coating provides a window for detection purposes. Samples are injected into the capillary by a variety of means, e.g. electrophoretic loading or displacement. In the former, the inlet end of the capillary is immersed in the sample and a pulse of high voltage is applied. The displacement method involves forcing the sample into the capillary, either by applying pressure in the sample vial using an inert gas, or by introducing a vacuum at the outlet. The detectors used in CE are similar to those used in chromatography, e.g. UV/visible spectrophotometric systems. Fluorescence detection is more sensitive, but this may require sample derivitization. Electrochemical and conductivity detection are also used in some applications, e.g. conductivity detection of inorganic cations such as Na+ , K+.
EOF, described above, is essential to the most commonly used types of CE. The existence of EOF in the capillary is the result of the net negative charge on the fused silica surface at pH values over 3.0. The resulting solvent flow towards the cathode is greater than the
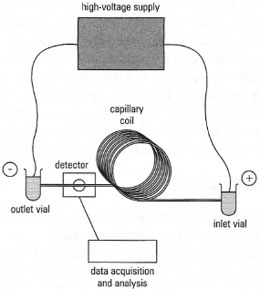 |
| Fig 33.3 Components of a capillary electrophoresis system. |




