Electroanalytical techniques
Electrochemical methods are used to quantify a broad range of different molecules, including ions, gases, metabolites and drugs.*Note: The basis of all electrochemical analysis is the transfer of electrons from one atom or molecule to another atom or molecule in an obligately coupled oxidation-reduction reaction la redox reaction).
It is convenient to separate such redox reactions into two half-reactions and, by convention, each is written as:
| oxidized form + electron(s) (ne−) | reduction | reduced form |
| oxidation |
You should note that the half-reaction is reversible: by applying suitable conditions, reduction or oxidation can take place. As an example, a simple redox reaction occurs when metallic zinc (Zn) is placed in a solution containing copper ions (Cu), as follows:
| ⇒ Equation [34.2] | Cu2+ + Zn → Cu + Zn2+ |
By convention, the electrode potential of any half-reaction is expressed relative to that of a standard hydrogen electrode (half-reaction 2H+ + 2e− → H2) and is called the standard electrode potential, E0. Table 34.1 shows the values of E0 for selected half-reactions. With any pair of half-reactions from this series, electrons will flow from that having the lowest electrode potential to that of the highest. E0 is determined at pH = 0. It is often more appropriate to express standard electrode potentials at pH 7 for biological systems, and the symbol E0' is used: in all circumstances, it is important that the pH is dearly stated.
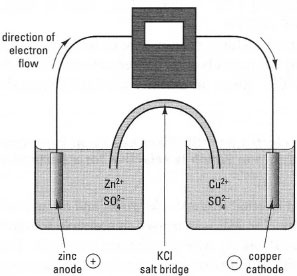 |
| Fig. 34.1 A simple galvanic electrochemical cell. The KCI salt bridge allows migration of ions between the two compartments but prevents mixing of the two solutions. |
- excellent detection limits, and wide operating range (10−1 to 10−8 mol L−1);
- measurements may be made on very small volumes (µL) allowing small amounts (pmol) of sample to be measured in some cases;
- miniature electrochemical sensors can be used for certain in vivo measurements, e.g. pH, glucose, oxygen content.
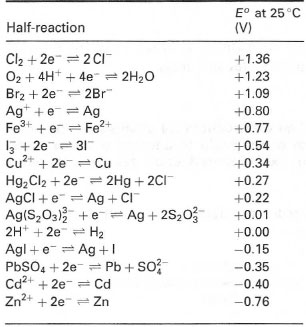 |
| Table 34.1 Standard electrode potentials* (E0) for selected half-reactions |




