Radioactive decay
There are three forms of radioactivity (Table 35.1) arising from three main types of nuclear decay: |
| Table 35.1 Types of radioactivity and their properties |
**Distance at which radiation intensity is reduced to half.
- Alpha decay involves the loss of a particle equivalent to a helium nucleus. Alpha (α) particles, being large and positively charged, do not penetrate far in living tissue, but they do cause ionization damage and this makes them generally unsuitable for tracer studies.
- Beta decay involves the loss or gain of an electron or its positive
counterpart, the positron. There are three sub-types:
- Negatron (β−) emission: loss of an electron from the nucleus when a neutron transforms into a proton. Examples of negatron-emitting isotopes are: 3H, 14C, 32P, 45Ca and 60Co.
- Positron (β−) emission: loss of a positron when a proton transforms into a neutron. This only occurs when sufficient energy is available from the transition and may involve the production of gamma rays when the positron is later annihilated by collision with an electron.
- Electron capture (EC): when a proton 'captures' an electron and transforms into a neutron. This may involve the production of x-rays as electrons 'shuffle' about in the atom (as with 125I) and it frequently involves electron emission.
- Internal transition involves the emission of electromagnetic radiation in the form of gamma (γ) rays from a nucleus in a met astable state and always follows initial alpha or beta decay. Emission of gamma radiation leads to no further change in atomic number or mass.
Each radioactive particle or ray carries energy, usually measured in electron volts (eV). The particles or rays emitted by a particular radioisotope exhibit a range of energies, termed an energy spectrum, characterized by the maximum energy of the radiation produced, Emax (Table 35.2).
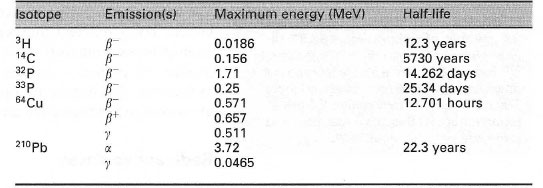 |
| Table 35.2 Properties of selected isotopes |
The energy spectrum of a particular radioisotope is relevant to the following:
- Safety: isotopes with the highest maximum energies will have the greatest penetrating power, requiring appropriate shielding (Table 35.1).
- Detection: different instruments vary in their ability to detect isotopes with different energies.
- Discrimination: some instruments can distinguish between isotopes, based on the energy spectrum of the radiation produced.
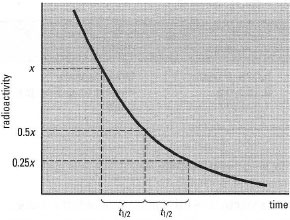 |
| Fig. 35. 1 Decay of a radioactive isotope with time. The time taken for the radioactivity to decline from × to 0.5× is the same as the time taken for the radioactivity to decline from 0.5× to 0.25×, and so on. This time is the half-life (t½) of the isotope. |
To calculate the fraction (f) of the original radioactivity left after a particular time (t), use the following relationship:
| ⇒ Equation [35.1] | f = ex, where x = −0.693t/t½ |




