Liquid chromatography
The basic chromatographic system comprises a stationary phase (adsorbant), usually alumina, silica gel or cellulose, through which a mobile phase travels (elutes). Separation of a mixture of compounds is achieved by a combination of the differing 'adsorption' and solubility characteristics of the components on the stationary phase and in the mobile phase respectively.Liquid chromatography is used both as an analytical method to determine the complexity of mixtures and the purity of compounds, and as a preparative system for the separation of mixtures. Liquid chromatography is divided into two general types:
- Thin-layer chromatography (TLC): in which a glass or plastic plate is coated with a thin layer of the stationary phase and the mobile phase ascends the plate by capillary action. TLC is essentially an analytical tool and preparative TLC has been largely superseded by flash chromatography.
- Column chromatography: in which the stationary phase is packed into a glass column and the mobile phase is passed down the column, either by gravity (gravity chromatography) or under low pressure from a pump or nitrogen cylinder (flash chromatography). These are the preparative systems.
The essential components of a TLC system are:
- The stationary phase comprising the layer of adsorbant on a solid backing - the chromatoplate. Aluminium or plastic-backed chromatoplates are now the norm having replaced glass plates, which needed to be prepared 'inhouse'. The chromatoplates (20 cm × 20 cm) can be cut down to the more useful size (2 cm × 5 cm) for analytical work, using a guillotine. The adsorbant often contains a fluorescent compound (ZnS) to enable visualization of the compounds after elution.
- The development tank: for plates of (2 cm × 5 cm) a clean, dry beaker (l00 mL) covered with a watch-glass is ideal. The eluting solvent should be about 3mm deep and filter paper should be placed in the tank to saturate the tank atmosphere with solvent vapour (Fig. 32.11).
- The application system: a micropipette or a micro syringe to place the solution of the mixture on the chromatoplate. Micropipettes (Fig. 32.12) are the more common and Box 32.2 gives the instructions for their preparation.
- The eluent: finding the eluent, which will give the best separation of the components of the mixture, is by experiment - you may need to try several solvents of differing polarity (Table 32.1) or mixtures of solvents to find the best eluent.
- A visualization system to be able to see colourless separated components on the chromatogram. If the plate contains a fluorescer, it can be viewed under UV light (λ = 254 nm) in a special box or cabinet. The ZnS in the stationary phase fluoresces green, whereas the 'spots' of separated compounds appear dark. Alternatively, the plate can be placed in a sealed jar containing a few iodine crystals. The iodine vapour stains the plate light brown and the 'spots' dark brown.
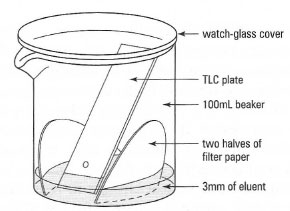 |
| Fig. 32.11 The developing tank for TLC. |
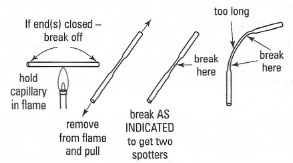 |
| Fig. 32.12 How to make micropipettes. |
 |
| Table 32.1 The elutropic series of solvents for chromatography |
You can express the movement of an individual compound up the TLC plate in terms of its Rf (relative frontal mobility) value, where:
⇒ Equation [31.4]
| Rf = | distance moved by compound/distance moved by solvent |
Column chromatography
This is used for the preparative scale separation of mixtures of compounds. There are many variations in detail of equipment and technique such as column type, packing, sample application and fraction collection, many of which are a matter of personal choice and apparatus available. Typical arrangements are shown in Fig. 32.15 and for a detailed description of all these variations you should consult the specialist texts such as Errington (1997), Harwood et al. (2000) and Furniss et al. (1989).
Gravity chromatography is used to separate the components of a mixture which have a difference in Rf value of at least 0.3. Flash chromatography, because of the smaller size of the adsorbant particles, is more effective separating mixtures components of ΔRf = 0.15 and is also faster.
*Note: Before attempting a preparative mixture separation by column chromatography, you must always analyse the mixture by TLC to establish the stationary phase and solvent parameters for effective separation and to determine the Rf values of the components.
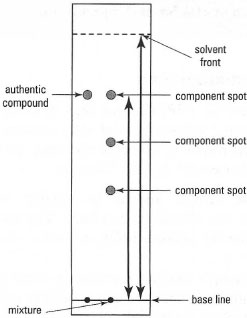 |
| Fig. 32.13 A thin-layer chromatogram. |
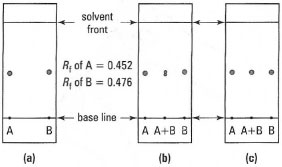 |
| Fig. 32.14 Double-spotting technique: (a) compounds A and B with close Rf values; (b) figure '8' of double spot shows that A and Bare different; (c) single spot for double spot shows that A and B could be the same. |
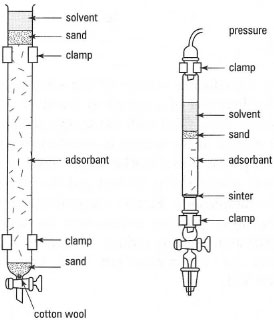 |
| Fig. 32.15 Column chromatography: (a) gravity chromatography; (b) flash chromatography. |




