Measuring radioactivity
The SI unit of radioactivity is the becquerel (Bq), equivalent to one disintegration per second (d.p.s.), but disintegrations per minute (d.p.m.) are also used. The curie (Ci) is a non-SI unit equivalent to the number of disintegrations produced by I g of radium (37 GBq). Table 35.3 shows the relationships between these units. In practice, most instruments are not able to detect all of the disintegrations from a particular sample, i.e. their efficiency is less than 100% and the rate of decay may be presented as counts min−1 (c.p.m.) or counts s−1 (c.p.s.). Most modern instruments correct for background radiation and inefficiencies in counting, converting count data to d.p.m. Alternatively, the results may be presented as the measured count rate, although this is only valid where the efficiency of counting does not vary greatly among samples.*Note: The specific activity is a measure of the quantity of radioactivity present in a known amount of the substance:
⇒ Equation [35.2]
| specific activity = | radioactivity (Bq, Ci, d.p.m., etc.) | ||
| amount of substance (mol, g, etc.) |
This is an important concept in practical work involving radioisotopes, since it allows interconversion of disintegrations (activity) and amount of substance (see Box 35.1).
 |
| Table 35.3 Relationships between units of radioactivity |
Two SI units refer to doses of radioactivity and these are used when calculating exposure levels for a particular source. The sievert (Sv) is the amount of radioactivity giving a dose in man equivalent to 1 gray (Gy) of x-rays: 1Gy = an energy absorption of 1 J kg−1 The dose received in most biological experiments is a negligible fraction of the maximum permitted exposure limit. Conversion factors from older units are given in Table 35.3.
The most important methods of measuring radioactivity for chemical purposes are described below.
The Geiger-Milller (G-M) tube This operates by detecting radiation when it ionizes gas between a pair of electrodes across which a voltage has been applied. You should use a handheld Geiger-Muller tube for routine checking for contamination. It is not possible to detect low-energy β− and α particles as they are not able to penetrate the window of the tube. In addition, γ-rays (of medium to high energy) pass through the filling gas causing little ionization, and hence have low efficiency.
The scintillation counter
This operates by detecting the scintillations (fluorescence 'flashes') produced when radiation interacts with certain chemicals called fluors. In solid (or external) scintillation counters (often referred to as 'gamma counters') the radioactivity causes scintillations in a crystal of fluorescent material held close to the sample. This method is only suitable for radioisotopes producing penetrating radiation.
Liquid scintillation counters are mainly used for detecting beta decay. The sample is dissolved in a suitable solvent containing the fluor(s) - the 'scintillation cocktail'. The radiation first interacts with the solvent, and the energy from this interaction is passed to the fluors which produce detectable light. The scintillations are measured by photomultiplier tubes which turn the light pulses into electronic pulses, the magnitude of which is directly related to the energy of the original radioactive event. The spectrum of electronic pulses is thus related to the energy spectrum of the radioisotope.
Modern liquid scintillation counters use a series of electronic 'windows' to split the pulse spectrum into two or three components. This may allow more than one isotope to be detected in a single sample, provided their energy spectra are sufficiently different (Fig. 35.2). A complication of this approach is that the energy spectrum can be altered by pigments and chemicals in the sample, which absorb scintillations or interfere with the transfer of energy to the fluor; this is known as quenching (Fig. 35.2). Most instruments have computer-operated quench correction facilities (based on measurements of standards of known activity and energy spectrum) which correct for such changes in counting efficiency.
Many liquid scintillation counters treat the first sample as a 'background', subtracting whatever value is obtained from the subsequent measurements as part of the procedure for converting to d.p.m. If not, you will need to subtract the background count from all other samples. Make sure that you use an appropriate background sample, identical in all respects to your radioactive sample but with no added radioisotope, in the correct position within the machine. Check that the- background reading is reasonable (15- 30c.p.m.). Tips for preparing samples for liquid scintillation counting are given in Box 35.2.
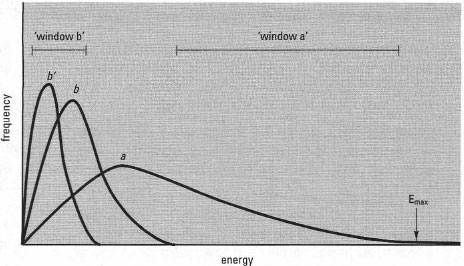 |
| Fig.35.2 Energy spectra for three radioactive samples, detected using a scintillation counter. Sample a is a high-energy beta emitter while b contains a low-energy beta emitter, giving a lower spectral range. Sample b' contains the same amount of low-energy beta emitter, but with quenching, shifting the spectral distribution to a lower energy band. The counter can be set up to record disintegrations within a selected range (a 'window'). Here, 'window a' could be used to count isotope a while 'window b' could give a value of isotope b, by applying a correction for the counts due to isotope a, based on the results from 'window a'. Dual counting allows experiments to be carried out using two isotopes (double labelling). |
γ-ray spectrometry
This is a method by which a mixture of γ-ray-emitting radionuclides can be resolved quantitatively by pulse-height analysis. It is based on the fact that pulse heights (voltages) produced by a photomuItiplier tube are proportional to the amounts of γ-ray energy arriving at the scintillant or a lithium-drifted germanium detector. The lithium-drifted germanium detector, which is abbreviated to Ge(Li) - pronounced 'jelly' provides higher resolution (narrower peaks), essential in the analysis of complex mixtures.
Autoradiography
This is a method where photographic film is exposed to the isotope. It is used mainly to locate radioactive tracers in thin sections of an organism or on chromatography papers and gels, but quantitative work is possible. The radiation interacts with the film in a similar way to light, silver grains being formed in the developed film where the particles or rays have passed through. The radiation must have enough energy to penetrate into the film, but if it has too much energy the grain formation may be too distant from the point where the isotope was located to identify precisely the point of origin (e.g. high-energy beta-emitters). Autoradiography is a relatively specialized method and individual lab protocols should be followed for particular isotopes/applications.




