Chromatography - introduction
Chromatography is used to separate the individual components of a mixture on the basis of differences in their physical characteristics, e.g. molecular size, shape, charge, volatility, solubility and/or adsorption properties. The essential components of a chromatographic system are:- A stationary phase, where a solid, a gel or an immobilized liquid is held by a support matrix.
- A chromatographic bed: the stationary phase may be packed into a glass or metal column, spread as a thin layer on a sheet of glass or plastic, or adsorbed on cellulose fibres (paper).
- A mobile phase, either a liquid or a gas which acts as a solvent, carrying the sample through the stationary phase and eluting from the chromatographic bed.
- A delivery system to pass the mobile phase through the chromatographic bed.
- A detection system to visualize the test substances.
*Note: In a chromatographic system, those substances which interact strongly with the stationary phase will be retarded to the greatest extent, while those which show little interaction will pass through with minimal delay, leading to differences in distances travelled or elution times.
Chromatography is sub-divided according to the mechanism of interaction of the solute with the stationary phase.
Adsorption chromatography
This is a form of solid-liquid chromatography. The stationary phase is a porous, finely divided solid which adsorbs molecules of the mixture on its surface by dipole-dipole interactions, hydrogen bonding and/or van der Waals' interactions (Fig. 31.1). The range of adsorbents is limited to polystyrene-based resins for non-polar molecules and silica, aluminium oxide and calcium phosphate for polar molecules. Most adsorbents must be activated by heating to 110-120°Cbefore use, since their adsorptive capacity is significantly decreased if water is adsorbed on the surface. Adsorption chromatography can be carried out in column or thin-layer form, using a wide range of organic solvents.
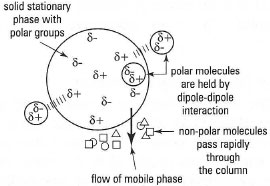 |
| Fig. 31.1 Adsorption chromatography (polar stationary phase). |
Partition chromatography
This is based on the partitioning of a substance between two liquid phases, in this instance the stationary and mobile phases. Substances which are more soluble in the mobile phase will pass rapidly through the system while those which favour the stationary phase will be retarded (Fig. 31.2). In normal phase partition chromatography the stationary phase is a polar solvent, usually water, supported by a solid matrix (e.g. cellulose fibres in paper chromatography) and the mobile phase is an immiscible, non-polar organic solvent. For reversed-phase partition chromatography the stationary phase is a non-polar solvent (e.g. a C18 hydrocarbon, such as octadecylsilane) which is chemically bonded to a porous support matrix (e.g. silica), while the mobile phase can be chosen from a wide range of polar solvents, usually water or an aqueous buffered solution containing one or more organic solvents, e.g. acetonitrile. Solutes interact with the stationary phase through non-polar interactions and so the least polar solutes elute last from the column. Solute retention and
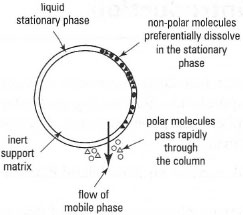 |
| Fig. 31.2 Liquid-liquid partition chromatography, e.g. reversed-phase HPLC. |
Ion-exchange chromatography (IEC)
Here, separations are carried out using a column packed with a porous matrix which has a large number of ionized groups on its surfaces, i.e. the stationary phase is an ion-exchange resin. The groups may be cation or anion exchangers, depending upon their affinity for positive or negative ions. The net charge on a particular resin depends on the pKa of the ionizable groups and the pH of the solution, in accordance with the Henderson-Hasselbalch equation.
For most practical applications, you should select the ion-exchange resin and buffer pH so that the test substances are strongly bound by electrostatic attraction to the ion-exchange resin on passage through the system, while the other components of the sample are rapidly eluted (Fig. 31.3). You can then elute the bound
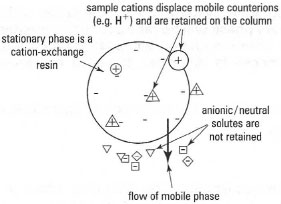 |
| Fig.31.3 Ion-exchange chromatography (cation exchanger). |
Computer-controlled gradient formers are available: if two or more components cannot be resolved using a linear salt gradient, an adapted gradient can be used in which the rate of change in salt concentration is decreased over the range where these components are expected to elute. IEC can be used to separate mixtures of a wide range of anionic and cationic compounds. Electrophoresis is an alternative means of separating charged molecules.
Gel permeation chromatography (GPC) or gel filtration Here, the stationary phase is in the form of beads of a cross-linked gel containing pores of a discrete size (Fig. 31.4). The size of the pores is controlled so that at the molecular level, the pores act as 'gates' that will exclude large molecules and admit smaller ones (Table 31.1). However, this gating effect is not an all-or-nothing phenomenon: molecules of intermediate size partly enter the pores. A column packed with such beads will have within it two effective volumes that are potentially available to sample molecules in the mobile phase, i.e. Vi, the volume surrounding the beads and Vii, the volume within the pores. If a sample is placed at the top of such a column, the mobile phase will carry the sample components down the column, but at different rates according to their molecular size. A very large molecule will have access to all of Vi but to none of Vii, and will therefore elute in the minimum possible volume (the 'void volume', or V0, equivalent to VI)' A very small molecule will have access to all of Vi and all of Vii, and therefore it has to pass through the total liquid volume of the column (Vt equivalent to Vi + Vii) before it emerges. Molecules of intermediate size have access to all of Vi but only part of Vii, and will elute at a volume between Vo and Vb in order of decreasing size depending on their access to Vii.
Cross-linked dextrans (e.g. Sephadex®), agarose (e.g. Sepharose®) and polyacrylamide (e.g. Bio-gel®) can be used to separate mixtures of macromolecules, particularly enzymes, antibodies and other globular proteins. Selectivity in GPC is solely dependent on the stationary phase, with the mobile phase being used solely to transport the sample components through
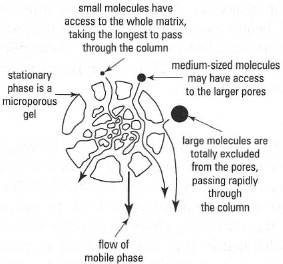 |
| Fig. 31.4 Gel permeation chromatography. |
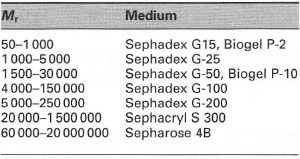 |
| Table 31.1 Fractionation ranges of selected GPC media |
Affinity chromatography
Affinity chromatography allows biomolecules to be purified on the basis of their biological specificity rather than by differences in physico-chemical properties, and a high degree of purification (more than l000-fold) can be expected. It is especially useful for isolating small quantities of material from large amounts of contaminating substances. The technique involves the immobilization of a complementary binding substance (the ligand) onto a solid matrix in such a way that the specific binding affinity of the ligand is preserved. When a biological sample is applied to a column packed with this affinity support matrix, the molecule of interest will bind specifically to the ligand, while contaminating substances will be washed through with the buffer (Fig. 31.5). Elution of the desired molecule can be achieved by changing the pH or ionic strength of the buffer, to weaken the non-covalent interactions between the molecule and the ligand, or by addition of other substances that have greater affinity for the ligand.
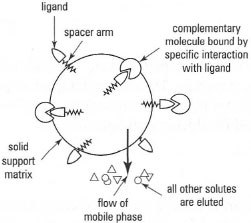 |
| Fig. 31.5 Affinity chromatography. |




