Potentiometry and ion-selective electrodes
Operating principlesThese systems involve galvanic cells and are based on measurement of the potential (voltage) difference between two electrodes in solution when no net current flows between them: no net electrochemical reaction occurs and measurements are made under equilibrium conditions. These systems include methods for measuring pH, ions, and gases such as CO2 and NH3. A typical potentiometric cell is shown in Fig. 34.2. It contains two electrodes:
- a 'sensing' electrode, the half-cell potential of which responds to changes in the activity (concentration) of the substance to be measured; the most common type of indicator electrodes are ion-selective electrodes (ISEs);
- a 'reference' electrode, the potential of which does not change, forming the second half of the cell.
Reference electrodes for potentiometry are of three main types:
- The standard hydrogen electrode, which is the reference half-cell electrode, defined as 0.0 V at all temperatures, against which values of E0 are expressed. H2 gas at 1 atmosphere pressure is bubbled over a platinum electrode immersed in an acid solution with an activity of unity. This electrode is rarely used for analytical work, since it is unstable and other reference electrodes are easier to construct and use.
- The calomel electrode (Fig. 34.3), which consists of a paste of mercury covered by a coat of calomel (Hg2Cl2), immersed in a saturated solution of KC!. The half-reaction Hg2Cl2 + 2e− → 2Hg + 2Cl− gives a stable standard electrode potential of +0.24 V.
- The silver/silver chloride electrode. This is a silver wire coated with AgCl and immersed in a solution of constant chloride concentration. The halfreaction AgCl + e− → Ag + Cl− gives a stable, standard electrode potential of +0.20 V.
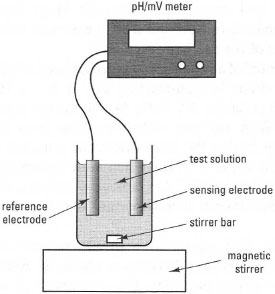 |
| Fig. 34.2 Components of a potentiometric cell. |
*Note: Ion-selective electrodes USEs) are based on measurement of a potential across a membrane which is selective for a particular analyte.
An ISE consists of a membrane, an internal reference electrode, and an internal reference electrolyte of fixed activity. The ISE is immersed in a sample solution that contains the analyte of interest, along with a reference electrode. The membrane is chosen to have a specific affinity for a particular ion, and if activity of this ion in the sample differs from that in the reference electrolyte, a potential develops across the membrane that is dependent on the ratio of these activities. Since the potentials of the two reference electrodes (internal and external) are fixed, and the internal electrolyte is of constant activity, the measured potential, E, is dependent on the membrane potential and is given by the Nernst equation:
⇒ Equation [34.3]
| E = | K + 2.303 | RT | log [a] | ||
| zF |
where K represents a constant potential which is dependent on the reference electrode, z represents the net charge on the analyte, [a] the activity of analyte in the sample and all other symbols and constants have their usual meaning. For a series of standards of known activity, a plot of E against log [a] should be linear over the working range of the electrode, with a slope of 2.303 RT/zF (0.059 V at 25°C).
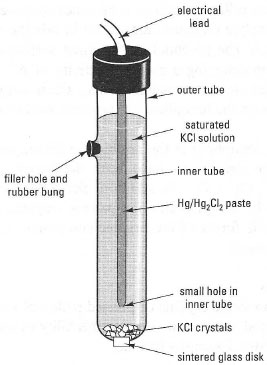 |
| Fig. 34.2 Components of a potentiometric cell. |
Although ISEs strictly measure activity, the potential differences can be approximated to concentration as long as (i) the analyte is in dilute solution, (ii) the ionic strength of the calibration standards matches that of the sample, e.g. by adding appropriate amounts of a high ionic strength solution to the standards, and (iii) the effect of binding to sample macromolecules (e.g. proteins, nucleic acids) is minimal. Potentiometric measurements on undiluted biological fluids, e.g. K+ and Na+ levels in plasma, tissue fluids or urine, are likely to give lower values than flame emission spectrophotometry, since the latter procedure measures total ion levels, rather than just those in aqueous solution.
All of the various types of membrane used in ISEs operate by incorporating the ion to be analysed into the membrane, with the accompanying establishment of a membrane potential. The scope of electrochemical analysis has been extended to measuring gases and non-ionic compounds by combining ISEs with gas-permeable membranes, enzymes, and even immobilized bacteria or tissues.
Glass membrane electrodes
The most widely used ISE is the glass membrane electrode for pH measurement. The membrane is thin glass (50 µm wall thickness) made of silica which contains some Na+. When the membrane is soaked in water, a thin hydrated layer is formed on the surface in which negative oxide groups (Si-O−) in the glass act as ion-exchange sites. If the electrode is placed in an acid solution, H+ exchanges with N+ in the hydrated layer, producing an external surface potential: in alkaline solution, H+ moves out of the membrane in exchange for N+. Since the inner surface potential is kept constant by exposure to a fixed activity of H+, a consistent, accurate potentiometric response is observed over a wide pH range. Glass electrodes for other cations (e.g. N+, N4+) have been developed by changing the composition of the glass, so that it is predominantly sensitive to the particular analyte, though the specificity of such electrodes is not absolute. The operating principles and maintenance of such electrodes are broadly similar to those for pH electrodes.
Gas-sensing glass electrodes
Here, an ISE in contact with a thin external layer of aqueous electrolyte (the 'filling solution') is kept close to the glass membrane by an additional, outer membrane that is selectively permeable to the gas of interest. The arrangement for a CO2 electrode is shown in Fig. 34.4: in this case the outer membrane is made of CO2-permeable silicone rubber. When CO2 gas in the sample selectively diffuses across the membrane and dissolves in the filling solution (in this case an aqueous NaHCO/NaCl mixture), a change in pH occurs owing to the shift in the equilibrium:
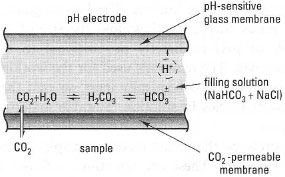 |
| Fig. 34.4 Underlying principles of a gassensing electrode. |
| ⇒ Equation [34.4] | CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3− |
The pH change is 'sensed' by the internal ion-selective pH electrode, and its response is proportional to the partial pressure of CO2 of the solution (PCO2). A similar principle operates in the NH3 electrode, where a Teflon® membrane is used, and the filling solution is NH4Cl.
Liquid and polymer membrane electrodes
In these types of ISEs, the liquid is a water-insoluble viscous solvent containing a soluble ionophore, i.e. an organic ion exchanger, or a neutral carrier molecule, that is specific for the analyte of interest. When this liquid is soaked into a thin membrane such as cellulose acetate, it becomes effectively immobilized. The arrangement of analyte (A+) and ionophore in relation to this membrane is shown in Fig. 34.5. The potential on the inner surface of the membrane is kept constant by maintaining a constant activity of A+ in the internal solution, so the potential change measured is that which results from A+ in the sample interacting with the ionophore in the outer surface of the membrane.
A relevant example of a suitable ionophore is the antibiotic valinomycin, which specifically binds K+. Other ionophores have been developed for measurement of, for example, NH4+, Ca2+,
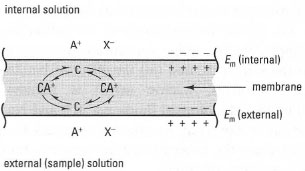 |
| Fig. 34.5 Underlying principles of a liquid membrane ion-selective electrode. A+ = analyte; C = neutral carrier ionophore;Em = surface potential; membrane potential = Emlinternal) - Em(external). |
Solid-state membrane electrodes
These contain membranes made from single crystals or pressed pellets of salts of the analyte. The membrane material must show some permeability to ions and must be virtually insoluble in water. Examples include:
- the fluoride electrode, which uses LaF3 impregnated with Eu2+ (the latter to increase permeability to F−). A membrane potential is set up when F− in the sample solution enters spaces in the crystal lattice;
- the chloride electrode, which uses a pressed pellet membrane of Ag2S and AgCl.




