Electrophoresis
Electrophoresis is a separation technique based on the movement of charged molecules in an electric field. Dissimilar molecules move at different rates and the components of a mixture will be separated when an electric field is applied. It is a widely used technique, particularly for the analysis of complex mixtures or for the verification of purity (homogeneity) of isolated molecules.While electrophoresis is mostly used for the separation of charged macromolecules, techniques are available for high-resolution separations, e.g. capillary electrophoresis, of small molecules such as amino acids, anions and catecholamines.
The electrophoretic mobility of a charged molecule depends on:
- Net charge - negatively charged molecules (anions) migrate towards the anode (+), while positively charged molecules (cations) migrate towards the cathode (-); highly charged molecules move faster towards the electrode of opposite charge than those with lesser charge.
- Size - frictional resistance exerted on molecules moving in a solution means that smaller molecules migrate faster than large molecules.
- Shape - the effect of friction also means that the shape of the molecule will affect mobility, e.g. globular proteins compared with fibrous proteins, linear surfactants compared with micellular surfactants.
- Electrical field strength - mobility increases with increasing field strength (voltage), but there are practical limitations to using high voltages, especially due to heating effects.
| ⇒ Equation [33.1] | µ = | qE | |
| r |
where q is the net charge on the molecule, r is the molecular radius and E is the field strength.
Most types of electrophoresis using supporting media are simple to carry out and the apparatus can be easily constructed, although inexpensive equipment is commercially available. High-resolution techniques such as twodimensional electrophoresis and capillary electrophoresis require more sophisticated equipment, both for separation and analysis (see later).
Simple electrophoretic separations can be performed either vertically (Fig. 33.1) or horizontally (Fig. 33.2). The electrodes are normally made of platinum wire, each in its own buffer compartment. In vertical electrophoresis, the buffer solution forms the electrical contact between the electrodes and the supporting medium in which the sample separation takes place. In horizontal electrophoresis electrical contact can be made by buffersoaked paper 'wicks' dipping in the buffer reservoir and laid upon the supporting medium. The buffer reservoir normally contains a divider acting as a barrier to diffusion (but not to electrical current), so that localized pH changes which occur in the region of the electrodes (as a result of electrolysis, are not transmitted to the supporting medium or the sample. Individual samples are spotted onto a solid supporting medium containing buffer or are applied to 'wells' formed in the supporting medium. The power pack used for most types of electrophoresis should be capable of delivering approximately 500V and approximately 100mA.
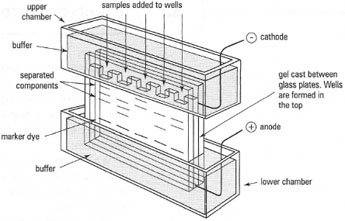 |
| Fig. 33.1 Apparatus for vertical slab electrophoresis (components move downwards from wells, through the gel matrix). |
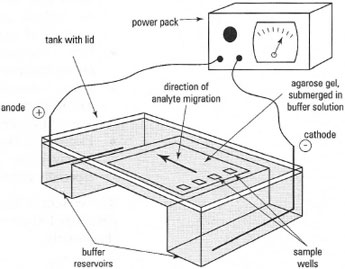 |
| Fig. 33.2 Agarose gel electrophoresis. |




