Fluorescence spectrophotometry
The principal components of a fluorescence spectrophotometer (fluorimeter) are shown in Fig. 26.4. The instrument contains two monochromators, one to select the excitation wavelength and the other to monitor the light emitted, usually at 90° to the incident beam (though light is actually emitted in all directions). As an example, the wavelengths used to measure the highly fluorescent compound naphthalene are 270 nm (excitation) and 340 nm (emission). Some examples of molecules with intrinsic fluorescence are given in Table 26.1.Compared with UV/visible spectrophotometry, fluorescence spectroscopy has certain advantages, including:
- Enhanced sensitivity (up to IOOO-fold),since the emitted light is detected against a background of zero, in contrast to spectrophotometry where small changes in signal are measured against a large 'background' (see eqn [26.5]).
- Increased specificity, because not one, but two, specific wavelengths are required for a particular compound.
- Not all compounds show intrinsic fluorescence, limiting its application. However, some non-fluorescent compounds may be coupled to fluorescent dyes, or fluorophores (e.g. alcohol ethoxylates may be coupled to naphthoyl chloride).
- The light emitted can be less than expected owing to quenching, i.e. when substances in the sample (e.g. oxygen) either interfere with energy transfer, or absorb the emitted light (in some instances, the sample molecules may self-quench if they are present at high concentration).
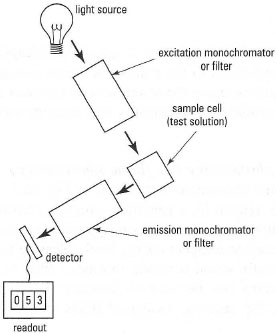 |
| Fig. 26.4 Components of a fluorimeter (fluorescence spectrophotometer). Note that sample cells for fluorimetry must have clear sides all round. |
 |
| Table 26.1 Examples of compounds with intrinsic fluorescence |
The sensitivity of fluorescence has made it invaluable in techniques in which specific chemicals, e.g. polycyclic aromatic hydrocarbons and alcohol ethoxylates, are linked to a fluorescent dye for detection in high-performance liquid chromatography.




