Fluorescence
With most molecules, after electrons are raised to a higher energy level by
absorption of electromagnetic radiation, they soon fall back to the ground
state by radiation1ess transfer of energy (heat) to the solvent. However, with
some molecules, the events shown in Fig. 26.3 may occur, i.e. electrons may
lose only part of their energy by non-radiant routes and the rest may be
emitted as electromagnetic radiation, a phenomenon known as fluorescence.
Since not all of the energy that was absorbed is emitted (due to non-radiant
loss), the wavelength of the fluorescent light is longer than the absorbed light
(longer wavelength = lower energy). Thus, a fluorescent molecule has both
an absorption spectrum and an emission spectrum.
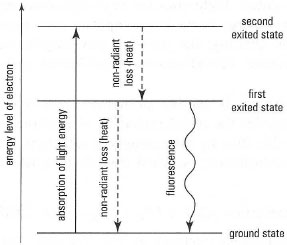 |
| Fig. 26.3 Energy levels and energy transitions
in fluorescence. |





