UV visible spectrophotometry
This is a widely used technique for measuring the absorption of radiation in the visible and UV regions of the spectrum. A spectrophotometer is an instrument designed to allow precise measurement at a particular wavelength, while a colorimeter is a simpler instrument, using filters to measure broader wavebands (e.g. light in the green, red or blue regions of the visible spectrum).Principles of light absorption
Two fundamental principles govern the absorption of light passing through a solution:
- The absorption of light is exponentially related to the number of molecules of the absorbing solute that are encountered, i.e. the solute concentration [C].
- The absorption of light is exponentially related to the length of the light path through the absorbing solution, l.
| ⇒ Equation [26.5] | log10 | ( | I0 | ) | = ε[C]l |
| I |
where ε is a constant for the absorbing substance at the wavelength the measurement is made and is termed the absorption coefficient or absorptivity, [C] is expressed as either mol L−1 or g L−1 and I is given in cm. This relationship is extremely useful, since most spectrophotometers are constructed to give a direct measurement of log10 (I0/I), termed the absorbance (A), or extinction (E), of a solution (older texts may use the outdated term optical density). Note that for substances obeying the Beer-Lambert relationship, A is linearly related to [C]. Absorbance at a particular wavelength is often shown as a subscript, e.g. A550 represents the absorbance at 550 nm. The proportion of light passing through the solution is known as the transmittance (T), and is calculated as the ratio of the emergent and incident light intensities.
Some instruments have two scales:
- An exponential scale from zero to infinity, measuring absorbance.
- A linear scale from 0 to 100, measuring (per cent) transmittance.
UV/visible spectrophotometer
The principal components of a UV/visible spectrophotometer are shown in Fig. 26.2. High-intensity tungsten bulbs are used as the light source in basic instruments, capable of operating in the visible region (i.e. 400-700nm). Deuterium lamps are used for DV spectrophotometry (200-400 nm); these lamps are fitted with quartz envelopes, since glass does not transmit UV radiation.
The spectrophotometer is a major improvement over the simple colorimeter since it uses a diffraction grating to produce a parallel
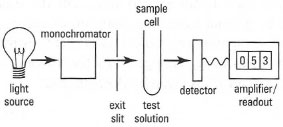 |
| Fig.26.2 Components of a UV/visible spectrophotometer. |
Bandwidth is affected by the width of the exit slit (the slit width), since the bandwidth will be reduced by decreasing the slit width. To obtain accurate data at a particular wavelength setting, the narrowest possible slit width should be used. However, decreasing the slit width also reduces the amount of light reaching the detector, decreasing the signal-to-noise ratio. The extent to which the slit width can be reduced depends upon the sensitivity and stability of the detection/amplification system and the presence of stray light.
Most UV/visible spectrophotometers are designed to take cells (cuvettes) with an optical path length of 10mm. Disposable plastic cells are suitable for routine work in the visible range using aqueous and alcohol-based solvents, while glass cells must be used for most other organic solvents. Glass cells are manufactured to more exacting standards, so you should use optically matched glass cells for accurate work, especially at low absorbances < 0.1), where any differences in the optical properties of cells for reference and test samples will be pronounced. Glass and plastic absorb UV light, so quartz cells must be used at wavelengths below 300nm.
Before taking a measurement, make sure that cells are clean, unscratched, dry on the outside, filled to the correct level and in the correct position in their sample holders. Unwanted material can accumulate on the inside faces of glass/quartz cells, so remove any deposits using acetone on a cotton bud, or soak overnight in 1 mol L−1 nitric acid. Corrosive and hazardous solutions must be used in cells with tightly fitting lids or Teflon® stoppers, to prevent damage to the instrument and to reduce the risk of accidental spillage.
Basic instruments use photocells similar to those used in simple colorimeters or photodiode detectors. In many cases, a different photocell must be used at wavelengths above and below 550-600nm, owing to differences in the sensitivity of such detectors over the visible waveband. The detectors used in more sophisticated instruments, give increased sensitivity and stability when compared with photocells.
Digital displays are increasingly used in preference to needle-type meters, as they are not prone to parallax errors and misreading of the absorbance scale. Some digital instruments can be calibrated to give a direct readout of the concentration of the test substance.
Types of U'Vlvisible spectrophotometer
Basic instruments are single-beam spectrophotometers in which there is only one light path. The instrument is set to zero absorbance using a blank solution, which is then replaced by the test solution, to obtain an absorbance reading. An alternative approach is used in double-beam spectrophotometers, where the light beam from the monochromator is split into two separate beams, one beam passing through the test solution and the other through a reference blank. Absorbance is then measured by an electronic circuit which compares the outputs from the reference (blank) and sample cells. Double-beam spectrophotometry reduces measurement errors caused by fluctuations in output from the light source or changes in the sensitivity of the detection system, since reference and test solutions are measured at the same time (Box 26.1). Recording spectrophotometers are double-beam instruments, designed for use with a chart recorder or computer, either by recording the difference in absorbance between reference and test solutions across a predetermined waveband to give an absorption spectrum, or by recording the change in absorbance at a particular wavelength as a function of time (e.g. in a kinetic determination.
Quantitative spectrophotometric analysis
A single (purified) substance in solution can be quantified using the Beer- Lambert relationship (eqn [26.5]), provided its absorptivity is known at a particular wavelength (usually at the absorption maximum for the substance, since this will give the greatest sensitivity). The molar absorptivity is the absorbance given by a solution with a concentration of 1 mol L−1 (= 1 kmol m−3) of the compound in a light path of 1cm. The appropriate value may be available from tabulated spectral data (e.g. Anon., 1963), or it can be determined experimentally by measuring the absorbance of known concentrations of the substance (Box 26.1) and plotting a standard curve. This should confirm that the relationship is linear over the desired concentration range and the slope of the line will give the molar absorptivity.




