Oxygen electrodes
The Clark (Rank) oxygen electrodeThese instruments measure oxygen in solution using the polarographic principle, i.e. by monitoring the current flowing between two electrodes when a voltage is applied. The most widespread electrode is the Clark type (Fig. 34.6), manufactured by Rank Bros, Cambridge, UK, which is suitable for measuring O2 concentrations in cell, organelle and enzyme suspensions. Pt and Au electrodes are in contact with a solution of electrolyte (normally saturated KCl). The electrodes are separated from the medium by a Teflon® membrane, permeable to O2. When a potential is applied across the electrodes, this generates a current proportional to the O2 concentration. The reactions can be summed up as:
| 4Ag → 4Ag+ + 4e− | (at silver anode) | |
| O2 + 2e− + 2H ↔ H2O2 | (in electrolyte solution; O2 replenished by diffusion from test solution) | |
| H2O2 + 2e− + 2H+ ↔ 2H2O | (at platinum cathode) |
Clark-type oxygen electrodes are also available in probe form for immersion in the test solution (Fig. 34.7) e.g, for field studies, allowing direct measurement of oxygen status in situ, in contrast to chemical assays. The main point to note is that
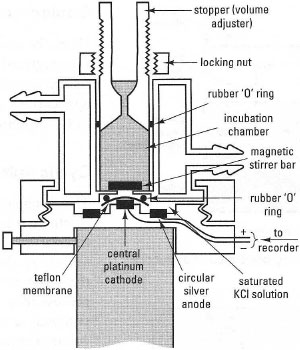 |
| Fig. 34.6 Transverse section through a Clark (Rank) oxygen electrode. |
The glucose electrode
The glucose electrode is a simple type of biosensor, whose basic design is shown in Fig. 34.8. It consists of a Pt electrode, overlaid by two membranes. Sandwiched between these membranes is a layer of the immobilized enzyme glucose oxidase. The outer membrane is glucose permeable and allows glucose in the sample to diffuse through to the glucose oxidase layer, where it is converted to gluconic acid and H2O2. The inner membrane is selectively permeable to H2O2, which is oxidized to O2 at the surface of the Pt electrode. The current arising from this release of electrons is proportional to the glucose concentration in the sample within the range 10−7 to 10−3 mol L−1.
Electrochemical detectors used in chromatography operate by voltammetric principles and currents are produced as the mobile phase flows over electrodes set at a fixed potential: to achieve maximum sensitivity, this potential must be set at a level that allows electrochemical reactions to occur in all analytes of interest.
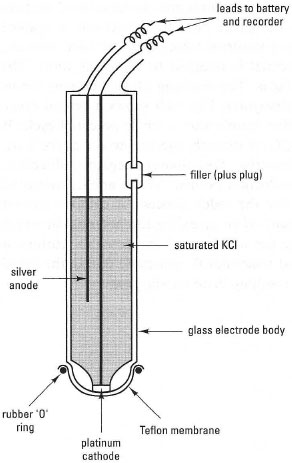 |
| Fig. 34.7 A Clark-type oxygen probe. |
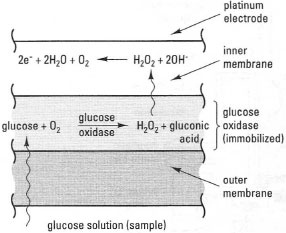 |
| Fig. 34.8 Underlying principles of a glucose electrode. |




